Abstract
Background
Aberrant immune responses play a key role in the pathogenesis of inflammatory bowel disease (IBD). Most studies conducted to delineate the underlying molecular mechanisms focus on adults; an understanding of these mechanisms in children remains to be determined. Here, cytokines and transcription factors produced by immune cells within the intestinal mucosa of pediatric patients stricken with ulcerative colitis (UC) and Crohn’s disease (CD) are characterized; potential diagnostic and therapeutic targets are identified.
Methods
Fifty-two pediatric IBD and non-IBD patients were enrolled in the study. Specimens were taken during ileocolonoscopy. Expression of 16 genes that encode cytokines or transcription molecules was determined by quantitative polymerase chain reaction. Clinical data were collected via retrospective chart review.
Results
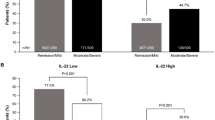
Overexpression of interleukin-17A (IL-17A) was evident in children with UC compared to both non-IBD and CD patients. IL-22 was strongly increased in UC patients only. Typical proinflammatory and immunoregulatory cytokines were pronounced in IBD patients, although to a lower extent in the latter case. Clustered gene expression enabled differentiation between UC and non-IBD patients.
Conclusion
Our findings highlight the crucial involvement of IL-17A immunity in the early course of IBD, particularly UC, and the potential value of gene panels in diagnosing pediatric IBD.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Burisch, J., Jess, T., Martinato, M. & Lakatos, P. L. The burden of inflammatory bowel disease in Europe. J. Crohns Colitis 7, 322–337 (2013).
Bene, L., Falus, A., Baffy, N. & Fulop, A. K. Cellular and molecular mechanisms in the two major forms of inflammatory bowel disease. Pathol. Oncol. Res. 17, 463 (2011).
Tegtmeyer, D., Seidl, M., Gerner, P., Baumann, U. & Klemann, C. Inflammatory bowel disease caused by primary immunodeficiencies—clinical presentations, review of literature, and proposal of a rational diagnostic algorithm. Pediatr. Allergy Immunol. 28, 412–429 (2017).
Winter, D. A. et al. Pediatric IBD-unclassified is less common than previously reported; results of an 8-year audit of the EUROKIDS Registry. Inflamm. Bowel Dis. 21, 2145–2153 (2015).
Levine, A. et al. ESPGHAN Revised Porto Criteria for the diagnosis of inflammatory bowel disease in children and adolescents. J. Pediatr. Gastroenterol. Nutr. 58, 795–806 (2014).
Fonseca-Camarillo, G. & Yamamoto-Furusho, J. K. Immunoregulatory pathways involved in inflammatory bowel disease. Inflamm. Bowel Dis. 21, 2188–2193 (2015).
Neurath, M. F. Cytokines in inflammatory bowel disease. Nat. Rev. Immunol. 14, 329–342 (2014).
Katsanos, K. H. & Papadakis, K. A. Inflammatory bowel disease: updates on molecular targets for biologics. Gut Liver 11, 455 (2017).
Yamamoto-Furusho, J. K. Inflammatory bowel disease therapy: blockade of cytokines and cytokine signaling pathways. Curr. Opin. Gastroenterol. 34, 187–193 (2018).
Maul, J. et al. Peripheral and intestinal regulatory CD4+CD25high T cells in inflammatory bowel disease. Gastroenterology 128, 1868–1878 (2005).
Asseman, C., Mauze, S., Leach, M. W., Coffman, R. L. & Powrie, F. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J. Exp. Med. 190, 995–1004 (1999).
Ruel, J., Ruane, D., Mehandru, S., Gower-Rousseau, C. & Colombel, J. F. IBD across the age spectrum: is it the same disease? Nat. Rev. Gastroenterol. Hepatol. 11, 88–98 (2014).
Hölttä, V. et al. Interleukin-17 immunity in pediatric Crohn disease and ulcerative colitis. J. Pediatr. Gastroenterol. Nutr. 57, 287–292 (2013).
Schmittgen, T. D. & Livak, K. J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 3, 1101–1108 (2008).
R Core Team. R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, Vienna, Austria, 2017).
Rosen, M. J. et al. Mucosal expression of type 2 and type 17 immune response genes distinguishes ulcerative colitis from colon-only Crohn’s disease in treatment-naive pediatric patients. Gastroenterology 152, 1345–1357.e1347 (2017).
Zhu, X. M., Shi, Y. Z., Cheng, M., Wang, D. F. & Fan, J. F. Serum IL-6, IL-23 profile and Treg/Th17 peripheral cell populations in pediatric patients with inflammatory bowel disease. Pharmazie 72, 283–287 (2017).
Elshal, M. F., Aldahlawi, A. M., Saadah, O. I. & McCoy, J. P. Reduced dendritic cells expressing CD200R1 in children with inflammatory bowel disease: correlation with Th17 and regulatory T cells. Int. J. Mol. Sci. 16, 28998–29010 (2015).
Fujino, S. et al. Increased expression of interleukin 17 in inflammatory bowel disease. Gut 52, 65–70 (2003).
Jiang, W. et al. Elevated levels of Th17 cells and Th17-related cytokines are associated with disease activity in patients with inflammatory bowel disease. Inflamm. Res. 63, 943–950 (2014).
Kobayashi, T. et al. IL23 differentially regulates the Th1/Th17 balance in ulcerative colitis and Crohn’s disease. Gut 57, 1682–1689 (2008).
Kaplan, M. et al. Are sTWEAK and IL-17A levels in inflammatory bowel disease associated with disease activity and etiopathogenesis? Inflamm. Bowel Dis. 22, 615–622 (2016).
Veldhoen, M. Interleukin 17 is a chief orchestrator of immunity. Nat. Immunol. 18, 612 (2017).
Frieder, J., Kivelevitch, D. & Menter, A. Secukinumab: a review of the anti-IL-17A biologic for the treatment of psoriasis. Ther. Adv. Chronic Dis. 9, 5–21 (2018).
Hueber, W. et al. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn’s disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut 61, 1693–1700 (2012).
Targan, S. R. et al. A randomized, double-blind, placebo-controlled phase 2 study of brodalumab in patients with moderate-to-severe Crohn’s disease. Am. J. Gastroenterol. 111, 1599 (2016).
Imam, T., Park, S., Kaplan, M. H. & Olson, M. R. Effector T helper cell subsets in inflammatory bowel diseases. Front. Immunol. 9, 1212 (2018).
Filtz, T. M., Vogel, W. K. & Leid, M. Regulation of transcription factor activity by interconnected post-translational modifications. Trends Pharmacol. Sci. 35, 76–85 (2014).
Monteleone, G. et al. Post-transcriptional regulation of Smad7 in the gut of patients with inflammatory bowel disease. Gastroenterology 129, 1420–1429 (2005).
Abraham, C., Dulai, P. S., Vermeire, S. & Sandborn, W. J. Lessons learned from trials targeting cytokine pathways in patients with inflammatory bowel diseases. Gastroenterology 152, 374–388 e374 (2017).
Atreya, R. & Neurath, M. F. Molecular pathways controlling barrier function in IBD. Nat. Rev. Gastroenterol. Hepatol. 12, 67 (2014).
Neurath, M. F. Current and emerging therapeutic targets for IBD. Nat. Rev. Gastroenterol. Hepatol. 14, 688 (2017).
Neurath, M. F. IL-23 in inflammatory bowel diseases and colon cancer. Cytokine Growth Factor Rev. 45, 1–8 (2019).
Chandradevan, R. et al. Evolution of pediatric inflammatory bowel disease unclassified (IBD-U): incorporated with serological and gene expression profiles. Inflamm. Bowel Dis. 24, 2285–2290 (2018).
Olsen, T., Goll, R., Cui, G., Christiansen, I. & Florholmen, J. TNF-alpha gene expression in colorectal mucosa as a predictor of remission after induction therapy with infliximab in ulcerative colitis. Cytokine 46, 222–227 (2009).
Atreya, R. & Neurath, M. F. Predicting therapeutic response by in vivo molecular imaging in inflammatory bowel diseases. Dig. Dis. 34, 552–557 (2016).
Buderus, S. et al. Inflammatory bowel disease in pediatric patients: characteristics of newly diagnosed patients from the CEDATA-GPGE Registry. Dtsch. Ärzteblatt Int. 112, 121–127 (2015).
Liu, Y., Beyer, A. & Aebersold, R. On the dependency of cellular protein levels on mRNA abundance. Cell 165, 535–550 (2016).
Kaplan, G. G. et al. The risk of developing Crohn’s disease after an appendectomy: a meta-analysis. Am. J. Gastroenterol. 103, 2925–2931 (2008).
Acknowledgements
Results shown in this study are part of the doctoral thesis of M.A.B. We thank Dr. Stephen H. Gregory (Providence, RI, USA) for his help writing and editing this manuscript. M.A.B. received a personal grant through the Sibylle Kalkhof-Rose Foundation.
Author information
Authors and Affiliations
Contributions
M.A.B. performed and analyzed experiments, interpreted the findings, and prepared the manuscript; R.L.K. supported acquisition of clinical data and critically revised the manuscript for important intellectual content; I.H. helped with the statistical analysis and graph editing; U.K and V.B. performed the endoscopies and collected tissue samples; A.D. performed tissue preparation; C.U.M., B.G., and L.P. supported the experiments and gave critical input to the project; F.Z. was involved in the study design and in the interpretation of the immunological findings; S.G. designed the study, acquired funding, collected tissue samples, and critically revised the manuscript for important intellectual content.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Busch, M.A., Gröndahl, B., Knoll, R.L. et al. Patterns of mucosal inflammation in pediatric inflammatory bowel disease: striking overexpression of IL-17A in children with ulcerative colitis. Pediatr Res 87, 839–846 (2020). https://doi.org/10.1038/s41390-019-0486-5
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41390-019-0486-5
This article is cited by
-
Predictive factors of the clinical efficacy of ustekinumab in patients with refractory Crohn’s disease: tertiary centers experience in Japan
International Journal of Colorectal Disease (2023)
-
Combined effect of vitamin C and vitamin D3 on intestinal epithelial barrier by regulating Notch signaling pathway
Nutrition & Metabolism (2021)