Abstract
Background
Neonatal sepsis is a leading cause of perinatal morbidity and mortality. In comparison to adults, neonates exhibit a higher susceptibility to infections. Myeloid-derived suppressor cells (MDSCs) are myeloid cells with suppressive activity on other immune cells accumulating during foetal life and controlling inflammation in neonates. Most studies investigating the mechanisms for MDSC-mediated immune suppression have been focused on T-cells. Thus far, little is known about the role of MDSC for monocyte function.
Methods
The impact of human cord blood MDSCs (CB-MDSCs) on monocytes was investigated in an in vitro model. CB-MDSCs were co-cultured with peripheral blood mononuclear cells and monocytes were analysed for expression of surface markers, T cell stimulatory and phagocytic capacity, as well as the production of intracellular cytokines by flow cytometry.
Results
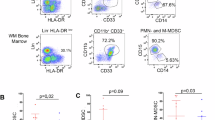
CB-MDSCs increased the expression of co-inhibitory molecules and decreased the expression of major histocompatibility complex class II molecules on monocytes, leading to an impaired T-cell stimulatory capacity. Upon bacterial stimulation, expression of phagocytosis receptors, phagocytosis rates and production of tumor necrosis factor-α by monocytes was diminished by CB-MDSCs.
Conclusion
We show that CB-MDSCs profoundly modulate monocyte functions, thereby indirectly impairing T-cell activation. Further research is needed to figure out if MDSCs could be a therapeutic target for inflammatory diseases in neonates like neonatal sepsis.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Stoll, B. J. et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics 126, 443–456 (2010).
Stichtenoth, G. et al. Major contributors to hospital mortality in very-low-birth-weight infants: data of the birth year 2010 cohort of the German Neonatal Network. Klin. Padiatr. 224, 276–281 (2012).
Dowling, D. J. & Levy, O. Ontogeny of early life immunity. Trends Immunol. 35, 299–310 (2014).
Sadeghi, K. et al. Immaturity of infection control in preterm and term newborns is associated with impaired toll-like receptor signaling. J. Infect. Dis. 195, 296–302 (2007).
Yan, S. R. et al. Role of MyD88 in diminished tumor necrosis factor alpha production by newborn mononuclear cells in response to lipopolysaccharide. Infect. Immun. 72, 1223–1229 (2004).
Orlikowsky, T. W. et al. Expression and regulation of B7 family molecules on macrophages (MPhi) in preterm and term neonatal cord blood and peripheral blood of adults. Cytom. B 53, 40–47 (2003).
Adkins, B. Peripheral CD4+ lymphocytes derived from fetal versus adult thymic precursors differ phenotypically and functionally. J. Immunol. 171, 5157–5164 (2003).
Gabrilovich, D. I. & Nagaraj, S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 9, 162–174 (2009).
Kostlin, N. et al. Granulocytic myeloid derived suppressor cells expand in human pregnancy and modulate T-cell responses. Eur. J. Immunol. 44, 2582–2591 (2014).
Rieber, N. et al. Neutrophilic myeloid-derived suppressor cells in cord blood modulate innate and adaptive immune responses. Clin. Exp. Immunol. 174, 45–52 (2013).
Schwarz, J. et al. Granulocytic myeloid-derived suppressor cells (GR-MDSC) accumulate in cord blood of preterm infants and remain elevated during the neonatal period. Clin. Exp. Immunol. 191, 328–337 (2017).
Kostlin, N. et al. Granulocytic myeloid derived suppressor cells from human cord blood modulate T-helper-cell response towards an anti-inflammatory phenotype. Immunology 152, 89–101 (2017).
Gille, C. et al. Monocytes derived from humanized neonatal NOD/SCID/IL2Rgamma(null) mice are phenotypically immature and exhibit functional impairments. Hum. Immunol. 73, 346–354 (2012).
Kostlin, N. et al. Granulocytic myeloid-derived suppressor cells accumulate in human placenta and polarize toward a Th2 phenotype. J. Immunol. 196, 1132–1145 (2016).
Kostlin, N. et al. Granulocytic myeloid-derived suppressor cells from human cord blood modulate T-helper cell response towards an anti-inflammatory phenotype. Immunology 152, 89–101 (2017).
Greten, T. F., Manns, M. P. & Korangy, F. Myeloid derived suppressor cells in human diseases. Int. J. Immunopharmacol. 11, 802–807 (2011).
Kostlin, N. et al. Granulocytic myeloid-derived suppressor cells (GR-MDSC) in breast milk (BM); GR-MDSC accumulate in human BM and modulate T-cell and monocyte function. Front. Immunol. 9, 1098 (2018).
He, Y. M. et al. Transitory presence of myeloid-derived suppressor cells in neonates is critical for control of inflammation. Nat. Med. 24, 224–231 (2018).
Ostrand-Rosenberg, S. et al. Frontline science: myeloid-derived suppressor cells (MDSCs) facilitate maternal-fetal tolerance in mice. J. Leukoc. Biol. 101, 1091–1101 (2017).
Grozdics, E. et al. B7 costimulation and intracellular indoleamine 2,3-dioxygenase expression in umbilical cord blood and adult peripheral blood. Biol. Blood Marrow Transplant. 20, 1659–1665 (2014).
Jones, C. A., Holloway, J. A. & Warner, J. O. Phenotype of fetal monocytes and B lymphocytes during the third trimester of pregnancy. J. Reprod. Immunol. 56, 45–60 (2002).
Rabe, H. et al. Staphylococcus aureus convert neonatal conventional CD4(+) T cells into FOXP3(+) CD25(+) CD127(low) T cells via the PD-1/PD-L1 axis. Immunology 141, 467–481 (2014).
Pagel, J. et al. Regulatory T cell frequencies are increased in preterm infants with clinical early-onset sepsis. Clin. Exp. Immunol. 185, 219–227 (2016).
Hartley, G. et al. Regulation of PD-L1 expression on murine tumor-associated monocytes and macrophages by locally produced TNF-alpha. Cancer Immunol. Immunother. 66, 523–535 (2017).
Roux, C. et al. Reactive oxygen species modulate macrophage immunosuppressive phenotype through the up-regulation of PD-L1. Proc. Natl Acad. Sci. USA. 116, 4326–4335 (2019).
Palojarvi, A. et al. Low monocyte HLA-DR expression as an indicator of immunodepression in very low birth weight infants. Pediatr. Res. 73, 469–475 (2013).
Juskewitch, J. E. et al. Monocyte HLA-DR expression and neutrophil CD64 expression as biomarkers of infection in critically ill neonates and infants. Pediatr. Res. 78, 683–690 (2015).
Gille, C. et al. Phagocytosis and postphagocytic reaction of cord blood and adult blood monocyte after infection with green fluorescent protein-labeled Escherichia coli and group B Streptococci. Cytom. B 76B, 271–284 (2009).
Gille, C. et al. Diminished phagocytosis-induced cell death (PICD) in neonatal monocytes upon infection with Escherichia coli. Pediatr. Res. 63, 33–38 (2008).
de Jong, E. et al. The phenotype and function of preterm infant monocytes: implications for susceptibility to infection. J. Leukoc. Biol. 102, 645–656 (2017).
Silveira-Lessa, A. L. et al. TLR expression, phagocytosis and oxidative burst in healthy and septic newborns in response to Gram-negative and Gram-positive rods. Hum. Immunol. 77, 972–980 (2016).
Leiber, A. et al. Neonatal myeloid derived suppressor cells show reduced apoptosis and immunosuppressive activity upon infection with Escherichia coli. Eur. J. Immunol. 47, 1009–1021 (2017).
Elahi, S. et al. Immunosuppressive CD71+ erythroid cells compromise neonatal host defence against infection. Nature 504, 158–162 (2013).
Hartel, C. et al. Cytokine responses correlate differentially with age in infancy and early childhood. Clin. Exp. Immunol. 142, 446–453 (2005).
Granland, C. et al. NOD1 and NOD2 expression and function in very preterm infant mononuclear cells. Acta Paediatr. 103, e212–e218 (2014).
Strunk, T. et al. Responsiveness of human monocytes to the commensal bacterium Staphylococcus epidermidis develops late in gestation. Pediatr. Res. 72, 10–18 (2012).
Wisgrill, L. et al. Reduced TNF-alpha response in preterm neonates is associated with impaired nonclassic monocyte function. J. Leukoc. Biol. 100, 607–612 (2016).
Semenzato, G. Tumour necrosis factor: a cytokine with multiple biological activities. Br. J. Cancer 61, 354–361 (1990).
David, J. M. et al. The IL-8/IL-8R axis: a double agent in tumor immune resistance. Vaccines 4, 22 (2016).
Alfaro, C. et al. Tumor-produced interleukin-8 attracts human myeloid-derived suppressor cells and elicits extrusion of neutrophil extracellular traps (NETs). Clin. Cancer Res. 22, 3924–3936 (2016).
Kostlin, N. et al. HLA-G promotes myeloid-derived suppressor cell accumulation and suppressive activity during human pregnancy through engagement of the receptor ILT4. Eur. J. Immunol. 47, 374–384 (2017).
Kostlin-Gille, N. et al. HIF-1alpha-deficiency in myeloid cells leads to a disturbed accumulation of myeloid derived suppressor cells (MDSC) during pregnancy and to an increased abortion rate in mice. Front. Immunol. 10, 161 (2019).
Pan, T. et al. Myeloid-derived suppressor cells are essential for maintaining feto-maternal immunotolerance via STAT3 signaling in mice. J. Leukoc. Biol. 100, 499–511 (2016).
Acknowledgements
This work has been supported by the research funds from the Medical Faculty of Tuebingen University (grant no. E.05.00433), the Federal Ministry of Education and Research (BMBF), the Ministry for Science, Research and Art of Baden-Württemberg (MWKBW), the European Social Fund (ESF) and the German Centre for Infection Research (DZIF).
Author information
Authors and Affiliations
Contributions
N.K.-G. and C.G. conceptualized and designed the study. N.K.-G., S.D., J.S., M.V., K.M. and B.S. performed experiments. N.K.-G., S.D., M.V. and C.G. analysed data. N.K.-G. drafted the initial manuscript. N.K.-G., T.W.O., C.F.P., F.W., U.H. and C.G. reviewed and revised the manuscript. All authors approved the final manuscript as submitted and agreed to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Dietz, S., Schwarz, J., Vogelmann, M. et al. Cord blood granulocytic myeloid-derived suppressor cells impair monocyte T cell stimulatory capacity and response to bacterial stimulation. Pediatr Res 86, 608–615 (2019). https://doi.org/10.1038/s41390-019-0504-7
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-019-0504-7
This article is cited by
-
Impaired functional capacity of polarised neonatal macrophages
Scientific Reports (2020)