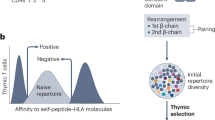
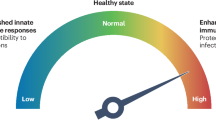
Abstract
Rare monogenetic diseases serve as natural models to dissect the molecular pathophysiology of the complex disease traits. Rheumatologic disorders by their nature are considered complex diseases with partially genetic origin, as illustrated by their heterogeneous genetic background and variable phenotypic presentation. Recent advances in genetic technologies have helped uncover multiple variants associated with disease susceptibility; however, a precise understanding of genotype–phenotype relationships is still missing. Inborn errors of immunity (IEIs), in addition to recurrent infections, may also present with autoimmune and autoinflammatory rheumatologic manifestations and have provided insights for understanding the underlying the principles of immune system homeostasis and mechanisms of immune dysregulation. This review discusses the rheumatologic manifestations in IEIs with overlapping and differentiating features in immunodeficiencies and rheumatologic disorders.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Eyre, S., Orozco, G. & Worthington, J. The genetics revolution in rheumatology: large scale genomic arrays and genetic mapping. Nat. Rev. Rheumatol. 13, 421–432 (2017).
Schmidt, R. E., Grimbacher, B. & Witte, T. Autoimmunity and primary immunodeficiency: two sides of the same coin? Nat. Rev. Rheumatol. 14, 7–18 (2017).
Okada, Y. et al. Genetics of rheumatoid arthritis contributes to biology and drug discovery. Nature 506, 376–381 (2014).
McIntosh, L. A. et al. Genome-wide association meta-analysis reveals novel juvenile idiopathic arthritis susceptibility loci. Arthritis Rheumatol. 69, 2222–2232 (2017).
Ombrello, M. J. et al. Genetic architecture distinguishes systemic juvenile idiopathic arthritis from other forms of juvenile idiopathic arthritis: clinical and therapeutic implications. Ann. Rheum. Dis. 76, 906–913 (2017).
Yasin, S. & Schulert, G. S. Systemic juvenile idiopathic arthritis and macrophage activation syndrome: update on pathogenesis and treatment. Curr. Opin. Rheumatol. 30, 514–520 (2018).
McAllister, K., Eyre, S. & Orozco, G. Genetics of rheumatoid arthritis: GWAS and beyond. Open Access Rheumatol. 3, 31–46 (2011).
Taylor, J. C. et al. Genome-wide association study of response to methotrexate in early rheumatoid arthritis patients. Pharmacogenomics J. 18, 528–538 (2018).
Fischer, A. et al. Autoimmune and inflammatory manifestations occur frequently in patients with primary immunodeficiencies. J. Allergy Clin. Immunol. 140, 1388.e8–1393.e8 (2017).
Liston, A., Enders, A. & Siggs, O. M. Unravelling the association of partial T-cell immunodeficiency and immune dysregulation. Nat. Rev. Immunol. 8, 545–558 (2008).
Notarangelo, L. D. Functional T cell immunodeficiencies (with T cells present). Annu. Rev. Immunol. 31, 195–225 (2013).
Puck, J. M. Newborn screening for severe combined immunodeficiency and T-cell lymphopenia. Immunol. Rev. 287, 241–252 (2019).
Henderson, L. A. et al. Expanding the spectrum of recombination-activating gene 1 deficiency: a family with early-onset autoimmunity. J. Allergy Clin. Immunol. 132, 969.e1-2–971.e1-2 (2013).
Kuijpers, T. W. et al. Idiopathic CD4+ T lymphopenia without autoimmunity or granulomatous disease in the slipstream of RAG mutations. Blood 117, 5892–5896 (2011).
Farmer, J. R. et al. Outcomes and treatment strategies for autoimmunity and hyperinflammation in patients with RAG deficiency. J. Allergy Clin. Immunol. Pract. 7, 1970–1985.e4 (2019).
Volk, T. et al. DCLRE1C (ARTEMIS) mutations causing phenotypes ranging from atypical severe combined immunodeficiency to mere antibody deficiency. Hum. Mol. Genet. 24, 7361–7372 (2015).
Whitmore, K. V. & Gaspar, H. B. Adenosine deaminase deficiency - more than just an immunodeficiency. Front. Immunol. 7, 314 (2016).
Gokturk, B. et al. CD3G gene defects in familial autoimmune thyroiditis. Scand. J. Immunol. 80, 354–361 (2014).
Rowe, J. H. et al. Patients with CD3G mutations reveal a role for human CD3gamma in Treg diversity and suppressive function. Blood 131, 2335–2344 (2018).
Poliani, P. L., Fontana, E., Roifman, C. M. & Notarangelo, L. D. zeta Chain-associated protein of 70 kDa (ZAP70) deficiency in human subjects is associated with abnormalities of thymic stromal cells: implications for T-cell tolerance. J. Allergy Clin. Immunol. 131, 597–600 e591-592 (2013).
Chan, A. Y. et al. A novel human autoimmune syndrome caused by combined hypomorphic and activating mutations in ZAP-70. J. Exp. Med. 213, 155–165 (2016).
Perniola, R. Twenty years of AIRE. Front. Immunol. 9, 98 (2018).
Constantine, G. M. & Lionakis, M. S. Lessons from primary immunodeficiencies: autoimmune regulator and autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy. Immunol. Rev. 287, 103–120 (2019).
Barzaghi, F. et al. Long-term follow-up of IPEX syndrome patients after different therapeutic strategies: an international multicenter retrospective study. J. Allergy Clin. Immunol. 141, 1036.e5–1049.e5 (2018).
Gambineri, E. et al. Clinical, immunological, and molecular heterogeneity of 173 patients with the phenotype of immune dysregulation, polyendocrinopathy, enteropathy, X-linked (IPEX) syndrome. Front. Immunol. 9, 2411 (2018).
Cepika, A. M. et al. Tregopathies: monogenic diseases resulting in regulatory T-cell deficiency. J. Allergy Clin. Immunol. 142, 1679–1695 (2018).
Vignoli, M. et al. CD25 deficiency: a new conformational mutation prevents the receptor expression on cell surface. Clin. Immunol. 201, 15–19 (2019).
Matsuoka, K. I. Low-dose interleukin-2 as a modulator of Treg homeostasis after HSCT: current understanding and future perspectives. Int. J. Hematol. 107, 130–137 (2018).
von Spee-Mayer, C. et al. Low-dose interleukin-2 selectively corrects regulatory T cell defects in patients with systemic lupus erythematosus. Ann. Rheum. Dis. 75, 1407–1415 (2016).
Schubert, D. et al. Autosomal dominant immune dysregulation syndrome in humans with CTLA4 mutations. Nat. Med. 20, 1410–1416 (2014).
Schwab, C. et al. Phenotype, penetrance, and treatment of 133 cytotoxic T-lymphocyte antigen 4-insufficient subjects. J. Allergy Clin. Immunol. 142, 1932–1946 (2018).
Slatter, M. A. et al. Hematopoietic stem cell transplantation for CTLA4 deficiency. J. Allergy Clin. Immunol. 138, 615.e1–619.e1 (2016).
Lo, B. et al. Autoimmune disease. Patients with LRBA deficiency show CTLA4 loss and immune dysregulation responsive to abatacept therapy. Science 349, 436–440 (2015).
Gamez-Diaz, L. et al. The extended phenotype of LPS-responsive beige-like anchor protein (LRBA) deficiency. J. Allergy Clin. Immunol. 137, 223–230 (2016).
Lo, B. et al. CHAI and LATAIE: new genetic diseases of CTLA-4 checkpoint insufficiency. Blood 128, 1037–1042 (2016).
Candotti, F. Clinical manifestations and pathophysiological mechanisms of the Wiskott-Aldrich Syndrome. J. Clin. Immunol. 38, 13–27 (2018).
Volpi, S. et al. A combined immunodeficiency with severe infections, inflammation, and allergy caused by ARPC1B deficiency. J. Allergy Clin. Immunol. 143, 2296–2299 (2019).
Gutierrez, M. et al. Partial growth hormone insensitivity and dysregulatory immune disease associated with de novo germline activating STAT3 mutations. Mol. Cell Endocrinol. 473, 166–177 (2018).
Fabre, A. et al. Clinical aspects of STAT3 gain-of-function germline mutations: a systematic review. J. Allergy Clin. Immunol. Pract. 7, 1958–1969.e9 (2019).
Mogensen, T. H. IRF and STAT transcription factors - from basic biology to roles in infection, protective immunity, and primary immunodeficiencies. Front. Immunol. 9, 3047 (2018).
Weinacht, K. G. et al. Ruxolitinib reverses dysregulated T helper cell responses and controls autoimmunity caused by a novel signal transducer and activator of transcription 1 (STAT1) gain-of-function mutation. J. Allergy Clin. Immunol. 139, 1629–1640 e1622 (2017).
Seidel, M. G. et al. The European Society for Immunodeficiencies (ESID) Registry working definitions for the clinical diagnosis of inborn errors of immunity. J. Allergy Clin. Immunol. Pract. 7, 1763–1770 (2019).
Odnoletkova, I. et al. The burden of common variable immunodeficiency disorders: a retrospective analysis of the European Society for Immunodeficiency (ESID) registry data. Orphanet J. Rare Dis. 13, 201 (2018).
Gutierrez, M. J., Sullivan, K. E., Fuleihan, R., Consortium, U. & Bingham, C. O. 3rd Phenotypic characterization of patients with rheumatologic manifestations of common variable immunodeficiency. Semin. Arthritis Rheum. 48, 318–326 (2018).
Jorgensen, S. F., Fevang, B. & Aukrust, P. Autoimmunity and inflammation in CVID: a possible crosstalk between immune activation, gut microbiota, and epigenetic modifications. J. Clin. Immunol. 39, 30–36 (2019).
El-Sayed, Z. A. et al. X-linked agammaglobulinemia (XLA): phenotype, diagnosis, and therapeutic challenges around the world. World Allergy Organ. J. 12, 100018 (2019).
Jhamnani, R. D., Nunes-Santos, C. J., Bergerson, J. & Rosenzweig, S. D. Class-switch recombination (CSR)/hyper-IgM (HIGM) syndromes and phosphoinositide 3-kinase (PI3K) defects. Front. Immunol. 9, 2172 (2018).
Azizi, G. et al. Autoimmunity in primary antibody deficiencies. Int. Arch. Allergy Immunol. 171, 180–193 (2016).
Kang, E. M. et al. Chronic granulomatous disease: overview and hematopoietic stem cell transplantation. J. Allergy Clin. Immunol. 127, 1319–1326 (2011). Quiz 1327–1328.
Dunogue, B. et al. Chronic granulomatous disease in patients reaching adulthood: a nationwide study in France. Clin. Infect. Dis. 64, 767–775 (2017).
Gardiner, G. J. et al. A role for NADPH oxidase in antigen presentation. Front. Immunol. 4, 295 (2013).
Errante, P. R., Perazzio, S. F., Frazao, J. B., da Silva, N. P. & Andrade, L. E. Primary immunodeficiency association with systemic lupus erythematosus: review of literature and lessons learned by the Rheumatology Division of a tertiary university hospital at Sao Paulo, Brazil. Rev. Bras. Reumatol. Engl. Ed. 56, 58–68 (2016).
Lopez-Lera, A. et al. Complement as a diagnostic tool in immunopathology. Semin. Cell Dev. Biol. 85, 86–97 (2019).
Ricklin, D., Reis, E. S. & Lambris, J. D. Complement in disease: a defence system turning offensive. Nat. Rev. Nephrol. 12, 383–401 (2016).
de Jesus, A. A., Canna, S. W., Liu, Y. & Goldbach-Mansky, R. Molecular mechanisms in genetically defined autoinflammatory diseases: disorders of amplified danger signaling. Annu. Rev. Immunol. 33, 823–874 (2015).
Ozen, S. & Bilginer, Y. A clinical guide to autoinflammatory diseases: familial Mediterranean fever and next-of-kin. Nat. Rev. Rheumatol. 10, 135–147 (2014).
Manukyan, G. & Aminov, R. Update on pyrin functions and mechanisms of familial mediterranean fever. Front. Microbiol. 7, 456 (2016).
Waite, A. L. et al. Pyrin and ASC co-localize to cellular sites that are rich in polymerizing actin. Exp. Biol. Med. (Maywood) 234, 40–52 (2009).
Xu, H. et al. Innate immune sensing of bacterial modifications of Rho GTPases by the Pyrin inflammasome. Nature 513, 237–241 (2014).
Sag, E., Bilginer, Y. & Ozen, S. Autoinflammatory diseases with periodic fevers. Curr. Rheumatol. Rep. 19, 41 (2017).
Hoang, T. K. & Albert, D. A. Novel presentations of periodic fever syndromes: discrepancies between genetic and clinical diagnoses. Eur. J. Rheumatol. 6, 12–18 (2019).
Laccetta, G., Tutera, M., Miccoli, M. & Consolini, R. Effects of anakinra on health-related quality of life in a patient with 1129G>A/928G>A mutations in MVK gene and heterozygosity for the mutation 2107C>A in CIAS1 gene. Front. Pediatr. 5, 128 (2017).
Drenth, J. P. et al. Mutations in the gene encoding mevalonate kinase cause hyper-IgD and periodic fever syndrome. International Hyper-IgD Study Group. Nat. Genet. 22, 178–181 (1999).
Hull, K. M. et al. The TNF receptor-associated periodic syndrome (TRAPS): emerging concepts of an autoinflammatory disorder. Medicine (Baltimore) 81, 349–368 (2002).
Canna, S. W. et al. An activating NLRC4 inflammasome mutation causes autoinflammation with recurrent macrophage activation syndrome. Nat. Genet. 46, 1140–1146 (2014).
Romberg, N. et al. Mutation of NLRC4 causes a syndrome of enterocolitis and autoinflammation. Nat. Genet. 46, 1135–1139 (2014).
Crow, Y. J. & Manel, N. Aicardi-Goutieres syndrome and the type I interferonopathies. Nat. Rev. Immunol. 15, 429–440 (2015).
Oda, H. & Kastner, D. L. Genomics, biology, and human illness: advances in the monogenic autoinflammatory diseases. Rheum. Dis. Clin. North Am. 43, 327–345 (2017).
Uggenti, C. & Crow, Y. J. Taking the STING out of inflammation. Nat. Rev. Rheumatol. 14, 508–509 (2018).
Hoeger, B., Serwas, N. K. & Boztug, K. Human NF-kappaB1 haploinsufficiency and Epstein-Barr virus-induced disease-molecular mechanisms and consequences. Front. Immunol. 8, 1978 (2017).
Dieguez-Gonzalez, R. et al. Analysis of TNFAIP3, a feedback inhibitor of nuclear factor-kappaB and the neighbor intergenic 6q23 region in rheumatoid arthritis susceptibility. Arthritis Res. Ther. 11, R42 (2009).
Vereecke, L., Beyaert, R. & van Loo, G. Genetic relationships between A20/TNFAIP3, chronic inflammation and autoimmune disease. Biochem. Soc. Trans. 39, 1086–1091 (2011).
Berteau, F. et al. Autosomic dominant familial Behcet disease and haploinsufficiency A20: a review of the literature. Autoimmun. Rev. 17, 809–815 (2018).
Zhang, Q., Lenardo, M. J. & Baltimore, D. 30 Years of NF-kappaB: a blossoming of relevance to human pathobiology. Cell 168, 37–57 (2017).
Damgaard, R. B. et al. OTULIN deficiency in ORAS causes cell type-specific LUBAC degradation, dysregulated TNF signalling and cell death. EMBO Mol. Med. 11, e9324 (2019). pii.
Navon Elkan, P. et al. Mutant adenosine deaminase 2 in a polyarteritis nodosa vasculopathy. N. Engl. J. Med. 370, 921–931 (2014).
Carmona-Rivera, C. et al. Deficiency of adenosine deaminase 2 triggers adenosine-mediated NETosis and TNF production in patients with DADA2. Blood 134, 395–406 (2019).
Ozen, S. What’s new in autoinflammation? Pediatr. Nephrol. https://doi.org/10.1007/s00467-018-4155-4 (2018).
Schepp, J. et al. Screening of 181 patients with antibody deficiency for deficiency of adenosine deaminase 2 sheds new light on the disease in adulthood. Arthritis Rheumatol. 69, 1689–1700 (2017).
Bousfiha, A. et al. The 2017 IUIS phenotypic classification for primary immunodeficiencies. J. Clin. Immunol. 38, 129–143 (2018).
Author information
Authors and Affiliations
Contributions
S.K.B., S.O. and K.B. wrote and revised the manuscript. J.P. prepared the figures and revised the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Köstel Bal, S., Pazmandi, J., Boztug, K. et al. Rheumatological manifestations in inborn errors of immunity. Pediatr Res 87, 293–299 (2020). https://doi.org/10.1038/s41390-019-0600-8
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-019-0600-8
This article is cited by
-
Genetic Disorders in Pediatric Rheumatology Clinic: When to Suspect, and Why?
Indian Journal of Pediatrics (2024)