Abstract
Background
Intraventricular hemorrhage (IVH) and post-hemorrhagic hydrocephalus (PHHC) remain major problems among premature infants. The need, timing and type of ventricular drainage are based on sonographic ventricular measures, without assessment of the dimensions of the frontal lobe. The aim of our study was to establish new reference values for sonographic frontal lobe cortico-ventricular thickness (FL-CVT) in a large cohort of infants.
Methods
All normal head ultrasound scans that were performed in our center during the first 4 days of life between January 2014 and December 2016 were retrospectively evaluated.
Results
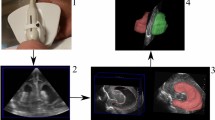
Scans were evaluated and plotted to create a reference range for the thickness of the frontal lobe in normal infants of 24–40 weeks’ gestation. The FL-CVT increased significantly during gestation. Calculating the area under the curve of the FL-CVT in 9 infants with post-hemorrhagic-hydrocephalus (PHHC) reveals a 20% mean loss of FL-CVT. The impact of increasing ventricular dilatation and of the various ventricular drainage procedures on the frontal lobe growth were described in two infants demonstrating the potential clinical value of this tool.
Conclusions
Head ultrasound provides a simple, non-invasive method for measuring the thickness of the frontal lobe, which grows significantly between 24 and 40 weeks’ gestation. In premature infants with PHHC, we suggest the use of the FL-CVT measure, in addition to ventricular size measures, as a direct assessment of the impact of the enlarged ventricles on the surrounding brain parenchyma. This could assist in the management of PHHC and determine the need and optimal timing for intervention.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Adams-Chapman, I., Hansen, N. I., Stoll, B. J. & Higgins, R. NICHD Research Network. Neurodevelopmental outcome of extremely low birth weight infants with post hemorrhagic hydrocephalus requiring shunt insertion. Pediatrics 121, e1167–e1177 (2008).
Hüppi, P. S. et al. Quantitative magnetic resonance imaging of brain development in premature and mature newborns. Ann. Neurol. 43, 224–235 (1998).
Mewes, A. U. et al. Regional brain development in serial magnetic resonance imaging of low-risk preterm infants. Pediatrics 118, 23–33 (2006).
Peterson, B. S. et al. Regional brain volumes and their later neurodevelopmental correlates in term and preterm infants. Pediatrics 111, 939–948 (2003).
Srinivasan, L. et al. Quantification of deep gray matter in preterm infants at term-equivalent age using manual volumetry of 3-tesla magnetic resonance images. Pediatrics 119, 759–765 (2007).
Thompson, D. K. et al. Perinatal risk factors altering regional brain structure in the preterm infant. Brain 130, 667–677 (2007).
Jary, S., De Carli, A., Ramenghi, L. A. & Whitelaw, A. Impaired brain growth and neurodevelopment in preterm infants with posthaemorrhagic ventricular dilatation. Acta Paediatr. 101, 743–748 (2012).
Levene, M. I. Measurement of the growth of the lateral ventricles in preterm infants with real-time ultrasound. Arch. Dis. Child. 56, 900–904 (1981).
Davies, M. W., Swaminathan, M., Chuang, S. L. & Betheras, F. R. Reference ranges for the linear dimensions of the intracranial ventricles in preterm neonates. Arch. Dis. Child. Fetal Neonatal Ed. 82, F218–F223 (2000).
Sondhi, V., Gupta, G., Gupta, P. K., Patnaik, S. K. & Tshering, K. Establishment of nomograms and reference ranges for intra-cranial ventricular dimensions and ventriculo-hemispheric ratio in newborns by ultrasonography. Acta Paediatr. 97, 738–744 (2008).
Brouwer, M. J. et al. New reference values for the neonatal cerebral ventricles. Radiology 262, 224–233 (2012).
Persutte, W. H., Coury, A. & Hobbins, J. C. Correlation of fetal frontal lobe and transcerebellar diameter measurements: the utility of a new prenatal sonographic technique. Ultrasound Obstet. Gynecol. 10, 94–97 (1997).
Fenton, T. R. A new growth chart for preterm babies: Babson and Benda’s chart updated with recent data and a new format. BMC Pediatr. 3, 13 (2003).
Makhoul, I. R. et al. Sonographic biometry of the frontal lobe in normal and growth-restricted neonates. Pediatr. Res. 55, 877–883 (2004).
Gilmore, J. H. et al. Infant cerebral ventricle volume: a comparison of 3-D ultrasound and magnetic resonance imaging. Ultrasound Med. Biol. 27, 1143–1146 (2001).
Kishimoto, J. et al. 3D ultrasound system to investigate intraventricular hemorrhage in preterm neonates. Phys. Med. Biol. 58, 7513–7526 (2013).
Del Bigio, M. R., Kanfer, J. N. & Zhang, Y. W. Myelination delay in the cerebral white matter of immature rats with kaolin-induced hydrocephalus is reversible. J. Neuropathol. Exp. Neurol. 56, 1053–1066 (1997).
Van Alfen-van der Velden, A. A. et al. Cerebral hemodynamics and oxygenation after serial CSF drainage in infants with PHVMD. Brain Dev. 29, 623–629 (2007).
Savman, K., Nilsson, U. A., Blennow, M., Kjellmer, I. & Whitelaw, A. Non-protein-bound iron is elevated in cerebrospinal fluid from preterm infants with posthemorrhagic ventricular dilatation. Pediatr. Res. 49, 208–212 (2001).
Soul, J. S., Eichenwald, E., Walter, G., Volpe, J. J. & du Plessis, A. J. CSF removal in infantile posthemorrhagic hydrocephalus results in significant improvement in cerebral hemodynamics. Pediatr. Res. 55, 872–876 (2004).
Singer, O. C. et al. MR volumetric changes after diagnostic CSF removal in normal pressure hydrocephalus. J. Neurol. 259, 2440–2446 (2012).
Brouwer, A. J. et al. European perspective on the diagnosis and treatment of posthaemorrhagic ventricular dilatation. Arch. Dis. Child. Fetal Neonatal Ed. 97, F50–F55 (2012).
de Vries, L. S. et al. Treatment thresholds for intervention in posthaemorrhagic ventricular dilation: a randomised controlled trial. Arch. Dis. Child. Fetal Neonatal Ed. 104, F70–F75 (2019).
Author information
Authors and Affiliations
Contributions
All authors take responsibility for the reported findings and have participated in the concept and design, analysis and interpretation of data, drafting or revising, and approval of this manuscript as submitted.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
About this article
Cite this article
Borenstein-Levin, L., Makhoul, S., Ilivitzki, A. et al. Neonatal frontal lobe: sonographic reference values and suggested clinical use. Pediatr Res 87, 536–540 (2020). https://doi.org/10.1038/s41390-019-0605-3
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-019-0605-3