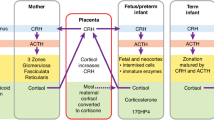
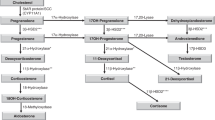
Abstract
Background
Most neonatal outcomes in neonates are related to normal adrenal gland function. Assessment of adrenal function in a sick preterm neonate remains a challenge, thus we hypothesized that adrenal steroid precursors to their product ratios have a direct relationship with neonatal outcomes.
Methods
We studied demographics of pregnancy and neonatal outcomes in 99 mother–infant pairs (24–41 weeks) and assayed 7 glucocorticoid precursors in the cortisol biosynthesis/degradation pathway. We correlated antenatal factors and short-term neonatal outcomes with these precursors and their ratios to assess maturity of individual enzymes.
Results
We found no correlation between cortisol levels with antenatal factors and outcomes. Antenatal steroid use impacted several cortisol precursors. 17-OH pregnenolone-to-cortisol ratio at birth was the best predictor of short-term neonatal outcomes, such as hypotension, RDS, IVH and PDA. A cord blood 17-OH pregnenolone:cortisol ratio of <0.21 predicts which neonate will have a normal outcome with a high sensitivity and specificity.
Conclusions
Maternal factors and antenatal steroids impact neonatal adrenal function and leads to maturation of adrenal function. 17-OH pregnenolone:cortisol ratio and not cortisol is the best predictor of adrenal function. Adrenal function can be assessed by evaluating the profile of adrenal steroids.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Watterberg, K. L. Adrenocortical function and dysfunction in the fetus and neonate. Semin. Neonatol. 9, 13–21 (2004).
Fernandez, E. F. & Watterberg, K. L. Relative adrenal insufficiency in the preterm and term infant. J. Perinatol. 29 (Suppl 2), S44–S49 (2009).
Speiser, P. W. et al. Congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency: an Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 103, 4043–4088 (2018).
Greaves, R. F. et al. Establishment of hormone reference intervals for infants born <30weeks’ gestation. Clin. Biochem. 47, 101–108 (2014).
Jobe, A. H. Glucocorticoids in perinatal medicine: misguided rockets? J. Pediatr. 137, 1–3 (2000).
Ishimoto, H. & Jaffe, R. B. Development and function of the human fetal adrenal cortex: a key component in the feto-placental unit. Endocr. Rev. 32, 317–355 (2011).
Hammer, G. D., Parker, K. L. & Schimmer, B. P. Minireview: Transcriptional regulation of adrenocortical development. Endocrinology 146, 1018–1024 (2005).
Watterberg, K. & Muglia, L. J. Fetal and Neonatal Physiology (Elsevier, Philadelphia, PA, 2017).
Miller, W. L. & Auchus, R. J. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr. Rev. 32, 81–151 (2011).
Tomlinson, J. W. et al. 11β-Hydroxysteroid dehydrogenase type 1: a tissue-specific regulator of glucocorticoid response. Endocr. Rev. 25, 831–866 (2004).
Benediktsson, R., Calder, A. A., Edwards, C. R. & Seckl, J. R. Placental 11 beta-hydroxysteroid dehydrogenase: a key regulator of fetal glucocorticoid exposure. Clin. Endocrinol. (Oxf.) 46, 161–166 (1997).
Green, B. B. et al. The role of placental 11-beta hydroxysteroid dehydrogenase type 1 and type 2 methylation on gene expression and infant birth weight. Biol. Reprod. 92, 149 (2015).
Karsli, T. et al. Assessment of neonatal adrenal size using high resolution 2D ultrasound and its correlation with birth demographics and clinical outcomes. J. Matern. Fetal Neonatal Med. 32, 377–383 (2017).
El-Khuffash, A., McNamara, P. J., Lapointe, A. & Jain, A. Adrenal function in preterm infants undergoing patent ductus arteriosus ligation. Neonatology 104, 28–33 (2013).
Huysman, M. W. A., Hokken-Koelega, A. C. S., Ridder, M. A. J. & Sauer, P. J. J. Adrenal function in sick very preterm infants. Pediatr. Res. 48, 629–633 (2000).
Coulter, C. L. et al. Functional maturation of the primate fetal adrenal in vivo. II. Ontogeny of corticosteroid synthesis is dependent upon specific zonal expression of 3 beta-hydroxysteroid dehydrogenase/isomerase. Endocrinology 137, 4953–4959 (1996).
Watterberg, K. L., Gerdes, J. S. & Cook, K. L. Impaired glucocorticoid synthesis in premature infants developing chronic lung disease. Pediatr. Res. 50, 190–195 (2001).
Aucott, S. W., Watterberg, K. L., Shaffer, M. L., Donohue, P. K. & PROPHET study group. Early cortisol values and long-term outcomes in extremely low birth weight infants. J. Perinatol. 30, 484 (2009).
Khashana, A., Ojaniemi, M., Leskinen, M., Saarela, T. & Hallman, M. Term neonates with infection and shock display high cortisol precursors despite low levels of normal cortisol. Acta Paediatr. 105, 154–158 (2016).
Khashana, A. et al. Cortisol precursors in neonates with vasopressor-resistant hypotension in relationship to demographic characteristics. J. Matern. Fetal Neonatal Med. 31, 2473–2477 (2018).
Soliman, A. T. et al. Circulating adrenocorticotropic hormone (ACTH) and cortisol concentrations in normal, appropriate-for-gestational-age newborns versus those with sepsis and respiratory distress: cortisol response to low-dose and standard-dose ACTH tests. Metabolism 53, 209–214 (2004).
Ng, P. C. et al. Transient adrenocortical insufficiency of prematurity and systemic hypotension in very low birthweight infants. Arch. Dis. Child. Fetal Neonatal Ed. 89, F119–F126 (2004).
Taylor, A. E., Keevil, B. & Huhtaniemi, I. T. Mass spectrometry and immunoassay: how to measure steroid hormones today and tomorrow. Eur. J. Endocrinol. 173, D1–D12 (2015).
Wynne-Edwards, K. E., Edwards, H. E. & Hancock, T. M. The human fetus preferentially secretes corticosterone, rather than cortisol, in response to intra-partum stressors. PLoS ONE 8, e63684 (2013).
Raubenheimer, P. J., Young, E. A., Andrew, R. & Seckl, J. R. The role of corticosterone in human hypothalamic-pituitary-adrenal axis feedback. Clin. Endocrinol. (Oxf.) 65, 22–26 (2006).
Aucott, S. W., Watterberg, K. L., Shaffer, M. L., Donohue, P. K. & Group, P. Do cortisol concentrations predict short-term outcomes in extremely low birth weight infants? Pediatrics 122, 775–781 (2008).
Khashana, A., Saarela, T., Ramet, M. & Hallman, M. Cortisol intermediates and hydrocortisone responsiveness in critical neonatal disease. J. Matern. Fetal Neonatal Med. 30, 1721–1725 (2017).
Anandi, V. S. & Shaila, B. Evaluation of factors associated with elevated newborn 17-hydroxyprogesterone levels. J. Pediatr. Endocrinol. Metab. 30, 677–681. (2017).
Tieh, P. Y., Yee, J. K., Hicks, R. A., Mao, C. S. & Lee, W. N. Utility of a precursor-to-product ratio in the evaluation of presumptive positives in newborn screening of congenital adrenal hyperplasia. J. Perinatol. 37, 283–287 (2017).
Seo, J. et al. Steroid profiling for congenital adrenal hyperplasia by tandem mass spectrometry as a second-tier test reduces follow-up burdens in a tertiary care hospital: a retrospective and prospective evaluation. J. Perinat. Med. 42, 121–127 (2013).
Hicks, R. A. et al. Precursor-to-product ratios reflect biochemical phenotype in congenital adrenal hyperplasia. Metabolomics 10, 123–131 (2014).
Karsli, T., Sutter, J. & Shekhawat, P. S. X-linked adrenal hypoplasia congenita due to NR0B1 (DAX1) deficiency presenting as severe respiratory distress in near term infants. Pediatr. Neonatol. 57, 444–445 (2016).
Fernandez, E. F., Montman, R. & Watterberg, K. L. ACTH and cortisol response to critical illness in term and late preterm newborns. J. Perinatol. 28, 797–802 (2008).
Hochwald, O., Holsti, L. & Osiovich, H. The use of an early ACTH test to identify hypoadrenalism-related hypotension in low birth weight infants. J. Perinatol. 32, 412–417 (2012).
Kwon, C. & Farrell, P. M. The magnitude and challenge of false-positive newborn screening test results. Arch. Pediatr. Adolesc. Med. 154, 714–718 (2000).
al Saedi, S., Dean, H., Dent, W. & Cronin, C. Reference ranges for serum cortisol and 17-hydroxyprogesterone levels in preterm infants. J. Pediatr. 126, 985–987 (1995).
Rauh, M. Steroid measurement with LC-MS/MS. Application examples in pediatrics. J. Steroid Biochem. Mol. Biol. 121, 520–527 (2010).
Millage, A. R., Latuga, M. S. & Aschner, J. L. Effect of perinatal glucocorticoids on vascular health and disease. Pediatr. Res. 81, 4–10 (2017).
Buyukkayhan, D., Ozturk, M. A., Kurtoglu, S., Koklu, E. & Yikilmaz, A. Effect of antenatal betamethasone use on adrenal gland size and endogenous cortisol and 17-hydroxyprogesterone in preterm neonates. J. Pediatr. Endocrinol. Metab. 22, 1027–1031 (2009).
Simard, J. et al. Molecular biology of the 3beta-hydroxysteroid dehydrogenase/delta5-delta4 isomerase gene family. Endocr. Rev. 26, 525–582 (2005).
Acknowledgements
We would like to express our gratitude to Dr. Samuel H Pepkowitz and Dr. Donald W Chandler from Endocrine Sciences Laboratory (LabCorp.) for their contribution in assaying our samples using the latest mass spectrometry technique in a blinded fashion. This study was internally funded by the Department of Pediatrics & Obstetrics & Gynecology, East Carolina University, Greenville, NC and steroid assays were performed by Endocrine Sciences (LabCorp) Laboratories.
Author information
Authors and Affiliations
Contributions
T.K. was responsible for conducting the study, data collection, data analysis, and writing initial draft of manuscript. V.G.J., Q.W., and M.M. were responsible for data analysis, preparing tables and figures, and helped write the initial draft of the manuscript. S.H.P. and D.W.C. did sample processing and assayed steroid hormones. P.S.S. was responsible for study design, data analysis, data interpretation, and wrote the final version of the manuscript. All authors approved the final version of the submitted manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Karsli, T., Jain, V.G., Mhanna, M. et al. Assessment of adrenal function at birth using adrenal glucocorticoid precursor to product ratios to predict short-term neonatal outcomes. Pediatr Res 87, 767–772 (2020). https://doi.org/10.1038/s41390-019-0629-8
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-019-0629-8