Abstract
Background
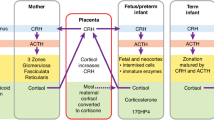
Adolescents born preterm have altered hypothalamic-pituitary-adrenal axis function with a blunted cortisol stress response, however, the influences of intrauterine growth restriction and race are unclear.
Methods
We measured salivary cortisol before and 20 min after a maximal-exercise stress test and calculated the cortisol stress response. We used linear regression to compare cortisol stress responses between preterm and term groups, adjusting for birth weight z-score and maternal hypertension, and examined effect modification by race and sex.
Results
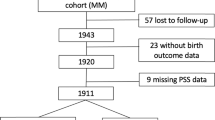
We evaluated 171 adolescents born preterm with very low birth weight and 50 born term. Adolescents born preterm had reduced cortisol stress response compared to term (0.03 vs. 0.08 μg/dL, p = 0.04). This difference was race dependent: non-Black adolescents born preterm had significantly reduced cortisol stress response compared to those born at term (adjusted β: −0.74; 95% CI −1.34, −0.15), while there was no difference in Black adolescents (0.53; −0.16, 1.22). Sex did not modify the relationship.
Conclusions
Adolescents born preterm exhibit a reduced salivary cortisol response to exercise stress, suggesting long-term alterations in the hypothalamic–pituitary–adrenal axis. This relationship was evident in non-Black but not in Black adolescents, suggesting that race may modify the influence of preterm birth on stress alterations of the hypothalamic–pituitary–adrenal axis.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Levine, S. Maternal and environmental influences on the adrenocortical response to stress in weanling rats. Science 156, 258–260 (1967).
Lee, M.-J. & Fried, S. K. The glucocorticoid receptor, not the mineralocorticoid receptor, plays the dominant role in adipogenesis and adipokine production in human adipocytes. Int. J. Obes. 38, 1228–1233 (2014).
Shively, C. A., Register, T. C. & Clarkson, T. B. Social stress, visceral obesity, and coronary artery atherosclerosis: product of a primate adaptation. Am. J. Primatol. 71, 742–751 (2009).
Whitworth, J. A., Williamson, P. M., Mangos, G. & Kelly, J. J. Cardiovascular consequences of cortisol excess. Vasc. Health Risk Manag. 1, 291 (2005).
Zellner, D. A. et al. Food selection changes under stress. Physiol. Behav. 87, 789–793 (2006).
Barker, D. J. Fetal programming of coronary heart disease. Trends Endocrinol. Metab. 13, 364–368 (2002).
Gitau, R., Fisk, N. & Glover, V. Human fetal and maternal corticotrophin releasing hormone responses to acute stress. Arch. Dis. Child. Fetal Neonatal Ed. 89, F29–F32 (2004).
Welberg, L. A., Thrivikraman, K. & Plotsky, P. M. Chronic maternal stress inhibits the capacity to up-regulate placental 11β-hydroxysteroid dehydrogenase type 2 activity. J. Endocrinol. 186, R7–R12 (2005).
Johnstone, J. F., Bocking, A. D., Unlugedik, E. & Challis, J. R. The effects of chorioamnionitis and betamethasone on 11β, hydroxysteroid dehydrogenase types 1 and 2 and the glucocorticoid receptor in preterm human placenta. J. Soc. Gynecol. Investig. 12, 238–245 (2005).
Duthie, L. & Reynolds, R. M. Changes in the maternal hypothalamic-pituitary-adrenal axis in pregnancy and postpartum: influences on maternal and fetal outcomes. Neuroendocrinology 98, 106–115 (2013).
Kelly, B. A. et al. Antenatal glucocorticoid exposure and long-term alterations in aortic function and glucose metabolism. Pediatrics 129, e1282–e1290 (2012).
Kajantie, E. et al. Birthsize, gestational age and adrenal function in adult life: studies of dexamethasone suppression and ACTH1-24 stimulation. Eur. J. Endocrinol. 149, 569–575 (2003).
Kajantie, E. et al. Body size at birth predicts hypothalamic-pituitary-adrenal axis response to psychosocial stress at age 60 to 70 years. J. Clin. Endocrinol. Metab. 92, 4094–4100 (2007).
Brummelte, S. et al. Cortisol levels in former preterm children at school age are predicted by neonatal procedural pain-related stress. Psychoneuroendocrinology 51, 151–163 (2015).
Winchester, S. B., Sullivan, M. C., Roberts, M. B. & Granger, D. A. Prematurity, birth weight, and socioeconomic status are linked to atypical diurnal hypothalamic–pituitary–adrenal axis activity in young adults. Res. Nurs. Health 39, 15–29 (2016).
Kapoor, A., Dunn, E., Kostaki, A., Andrews, M. H. & Matthews, S. G. Fetal programming of hypothalamo-pituitary-adrenal function: prenatal stress and glucocorticoids. J. Physiol. 572, 31–44 (2006).
Ter Wolbeek, M. et al. Neonatal glucocorticoid treatment: long-term effects on the hypothalamus-pituitary-adrenal axis, immune system, and problem behavior in 14-17 year old adolescents. Brain Behav. Immun. 45, 128–138 (2015).
Samuel, L. J., Roth, D. L., Schwartz, B. S., Thorpe, R. J. & Glass, T. A. Socioeconomic status, race/ethnicity, and diurnal cortisol trajectories in middle-aged and older adults. J. Gerontol. B Psychol. Sci. Soc. Sci. 73, 468–476 (2016).
Shaltout, H. A., Chappell, M. C., Rose, J. C. & Diz, D. I. Exaggerated sympathetic mediated responses to behavioral or pharmacological challenges following antenatal betamethasone exposure. Am. J. Physiol. Endocrinol. Metab. 300, E979–E985 (2011).
Washburn, L. K. et al. Antenatal corticosteroids and cardiometabolic outcomes in adolescents born with very low birth weight. Pediatr. Res. 82, 697 (2017).
Washburn, L., Nixon, P., Russell, G., Snively, B. M. & O’shea, T. M. Adiposity in adolescent offspring born prematurely to mothers with preeclampsia. J. Pediatr. 162, 912–917. e911 (2013).
Washburn, L. K., Nixon, P. A., Russell, G. B., Snively, B. M. & O’Shea, T. M. Preterm birth is associated with higher uric acid levels in adolescents. J. Pediatr. 167, 76–80 (2015).
South, A. M. et al. Association between preterm birth and the renin–angiotensin system in adolescence: influence of sex and obesity. J. Hypertens. 36, 2092 (2018).
Oken, E., Rich-Edwards, J., Kleinman, K. P. & Gillman, M. W. A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatr. 3, 6 (2003).
Kuczmarski, R. J. et al. CDC growth charts: United States. Adv. Data 8, 1–27 (2000).
Taylor, S. J. et al. Performance of a new pubertal self-assessment questionnaire: a preliminary study. Paediatr. Perinat. Epidemiol. 15, 88–94 (2001).
Godfrey, S. Exercise Testing in Children: Applications in Health and Disease (Saunders Limited, 1974).
Schwartz, E. B., Granger, D. A., Susman, E. J., Gunnar, M. R. & Laird, B. Assessing salivary cortisol in studies of child development. Child Dev. 69, 1503–1513 (1998).
Miller, R., Plessow, F., Rauh, M., Gröschl, M. & Kirschbaum, C. Comparison of salivary cortisol as measured by different immunoassays and tandem mass spectrometry. Psychoneuroendocrinology 38, 50–57 (2013).
Greenland, S., Pearl, J. & Robins, J. M. Causal diagrams for epidemiologic research. Epidemiology 10, 37–48 (1999).
Grunau, R. E. et al. Neonatal pain-related stress and NFKBIA genotype are associated with altered cortisol levels in preterm boys at school age. PLoS ONE 8, e73926 (2013).
Grunau, R. E. et al. Altered basal cortisol levels at 3, 6, 8 and 18 months in infants born at extremely low gestational age. J. Pediatr. 150, 151–156 (2007).
Buske-Kirschbaum, A. et al. Hypothalamic-pituitary-adrenal axis function and the cellular immune response in former preterm children. J. Clin. Endocrinol. Metab. 92, 3429–3435 (2007).
Kajantie, E. et al. Size at birth, gestational age and cortisol secretion in adult life: foetal programming of both hyper‐and hypocortisolism? Clin. Endocrinol. 57, 635–641 (2002).
Phillips, D. I. et al. Low birth weight predicts elevated plasma cortisol concentrations in adults from 3 populations. Hypertension 35, 1301–1306 (2000).
Kaseva, N. et al. Blunted hypothalamic‐pituitary‐adrenal axis and insulin response to psychosocial stress in young adults born preterm at very low birth weight. Clin. Endocrinol. 80, 101–106 (2014).
Cohen, S. et al. Socioeconomic status, race, and diurnal cortisol decline in the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Psychosom. Med. 68, 41–50 (2006).
VanderWeele, T. J. & Robinson, W. R. On causal interpretation of race in regressions adjusting for confounding and mediating variables. Epidemiology 25, 473 (2014).
DeSantis, A. S., Adam, E. K., Hawkley, L. C., Kudielka, B. M. & Cacioppo, J. T. Racial and ethnic differences in diurnal cortisol rhythms: are they consistent over time? Psychosom. Med. 77, 6–15 (2015).
McEwen, B. S. & Gianaros, P. J. Central role of the brain in stress and adaptation: links to socioeconomic status, health, and disease. Ann. NY Acad. Sci. 1186, 190–222 (2010).
Acknowledgements
We thank the participants and their families; Patricia Brown, research nurse; and Alice Scott, study coordinator. This study was funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (PO1 HD047584; HD084227), the Clinical Research Unit of Wake Forest Baptist Medical Center (MCRR/NIH MO1-RR07122), and the Forsyth Medical Center and Wake Forest School of Medicine Department of Pediatrics research funds.
Author contributions
C.L.B. conceptualized and designed the study, analyzed and interpreted the data, drafted the article, and gave final approval of the version to be published. K.M., A.M.S., H.A.S., and E.T.J. conceptualized and designed the study, revised the article critically important intellectual content, and gave final approval of the version to be published. P.A.N. and L.K.W. conceptualized and designed the study, contributed to data acquisition, revised the article critically important intellectual content, and gave final approval of the version to be published.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Brown, C.L., Myers, K., South, A.M. et al. Influence of race on the effect of premature birth on salivary cortisol response to stress in adolescents. Pediatr Res 87, 1100–1105 (2020). https://doi.org/10.1038/s41390-019-0682-3
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41390-019-0682-3