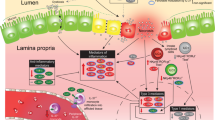
Abstract
Background
Necrotizing enterocolitis (NEC), a necrotic inflammation of the intestine, represents a major health problem in the very premature infant. Although prevention is difficult, the combination of ingestion of maternal-expressed breastmilk in conjunction with a probiotic provides the best protection. In this study, we establish a mechanism for breastmilk/probiotic protection.
Methods
Ultra-high-performance liquid chromatography-tandem mass spectrometry of Bifidobacterium longum subsp. infantis (B. infantis) secretions was used to identify an anti-inflammatory molecule. Indole-3-lactic acid (ILA) was then tested in an established human immature small intestinal cell line, necrotizing colitis enterocytes, and other immature human enteroids for anti-inflammatory effects and to establish developmental function. ILA was also examined in immature and mature enterocytes.
Results
We have identified ILA, a metabolite of breastmilk tryptophan, as the anti-inflammatory molecule. This molecule is developmentally functional in immature but not mature intestinal enterocytes; ILA reduces the interleukin-8 (IL-8) response after IL-1β stimulus. It interacts with the transcription factor aryl hydrocarbon receptor (AHR) and prevents transcription of the inflammatory cytokine IL-8.
Conclusions
This molecule produced by B. infantis (ATCC No. 15697) interaction with ingested breastmilk functions in a complementary manner and could become useful in the treatment of all at-risk premature infants for NEC if safety and clinical studies are performed.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Neu, J. & Walker, W. A. Necrotizing enterocolitis. N. Engl. J. Med. 364, 255–264 (2011).
Holman, R. C. et al. Necrotizing enterocolitis hospitalizations among neonates in the United States. Paediatr. Perinat. Epidemiol. 20, 498–506 (2006).
Claud, E. C. et al. Developmentally-regulated IκB expression in intestinal epithelium and susceptibility to flagellin-induced inflammation. Proc. Natl Acad. Sci. USA 101, 7404–7408 (2004).
Nanthakumar, N. et al. The mechanism of excessive intestinal inflammation in necrotizing enterocolitis: An immature innate immune response. PLoS ONE 6, e17776–e17785 (2011).
Weng, M. & Walker, W. A. The role of gut microbiota in programming the immune phenotype. J. Dev. Orig. Health Dis. 4, 203–214 (2013).
Groer, M. W., Gregory, K. E., Louis-Jacques, A., Thibeau, S. & Walker, W. A. The very low birth weight infant microbiome and childhood health. Birth Defects Res. Part C 105, 252–264 (2015).
Palmer, C., Bik, E. M., DiGiulio, D. B., Relman, D. A. & Brown, P. O. Development of the human infant intestinal microbiota. PLoS Biol. 5, e177–e185 (2007).
Nanthakumar, N., Fusunyan, R. D., Sanderson, I. R. & Walker, W. A. Inflammation in the developing human intestine: a possible pathophysiologic basis for necrotizing enterocolitis. Proc. Natl Acad. Sci. USA 97, 6043–6048 (2000).
Meng, D., Zhu, W., Ganguli, K., Shi, H. & Walker, W. A. Anti-inflammatory effects of Bifidobacterium longum subsp infantis secretion on immature human enterocytes are mediated by TLR4 receptors. Am. J. Physiol. Gastrointest. Liver Physiol. 311, G744–G753 (2016).
Gareau, M. G., Sherman, P. M. & Walker, W. A. Probiotics and the gut microbiota in intestinal health and disease. Nat. Rev. Gastroenterol. Hepatol. 7, 503–514 (2010).
O'Rourke, L. et al. Tryptophan metabolic profile in term and preterm breast milk: implications for health. J. Nutr. Sci. 7, e13 (2018).
Donnet-Hughes, A., Schriffin E. & Walker W. A. in Nutrition In Pediatrics Basic Science and Clinical Aspects. (eds Duggan, C., Koletzko, B., Watkins, J. & Walker, W. A.) 5th edn, 250–260 (Chinese Publications Inc., New Haven, CT, 2017).
Gregory, K. E. et al. Influence of maternal breast milk ingestion on acquisition of the intestinal microbiome in preterm infants. Microbiome 4, 68–78 (2016). (2016).
Jost, T., Lacroix, C., Braegger, C. P. & Chassard, C. New insights in gut microbiota establishment in healthy breast fed neonates. PLoS ONE 7, e44595–e44803 (2012).
Meng, D. et al. The toll-like receptor-4 in human and mouse colonic epithelium is developmentally regulated: a possible role in necrotizing enterocolitis. Pediatr. Res. 77, 416–424 (2015).
Repa, A. et al. Probiotics (Lactobacillus acidophilus and Bifidobacterium infantis) prevent NEC in VLBW infants fed breast milk but not formula. Pediatr. Res. 77, 381–388 (2015).
Cahill, C. et al. Differential expression of the activator protein 1 transcription factor regulates interleukin-1 beta induction of interleukin 6 in the developing enterocyte. PLos ONE 11, e0145184–e0145195 (2016).
Ganguli, K. et al. Probiotics prevent necrotizing enterocolitis by modulating enterocyte genes that regulate innate immune-mediated inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 304, G132–G141 (2013).
Guo, S. et al. Secreted metabolites of Bifidobacterium infantis and Lactobacillus acidophilus protect immature human enterocytes from IL-1β-induced inflammation: a transcription profiling analysis. PLoS ONE 10, e0124549–e0124557 (2015).
Sanderson, I. R. et al. Human immature enterocytes in vitro: modulation of the phenotype by extracellular matrix. Proc. Natl Acad. USA 93, 7717–7722 (1996).
Guo, S. et al. Secretions of Bifidobacteraium infantis and Lactobacillus acidophilus protect intestinal epithelial barrier function. J. Pediatr. Gastroenterol. Nutr. 64, 404–412 (2017).
Senger, S. et al. Human immature-derived enterospheres provide insights on intestinal development and a novel model to study necrotizing enterocolitis (NEC). Cell. Mol. Gastroenterol. Hepatol. J. 5, 549–568 (2018).
Lu, L., Li, T., William, G., Petit, E. & Walker, W. A. Hydrocortisone induces changes in gene expression and differentiation in immature human enterocytes. Am. J. Phys. 300, G425–G432 (2011).
Metidji, A. et al. Article: The environmental sensor AHR protects from inflammatory damage by maintaining intestinal stem cell homeostasis and barrier integrity. Immunity 49, 353–362 (2018).
Zhao, B., Degroot, D. E., Hayashi, A., He, G. & Denison, M. S. CH223191 is a ligand-selective antagonist of the Ah (Dioxin) receptor. Toxicol. Sci. 117, 393–403 (2010).
Theodoridis, G. A., Gika, H. G., Want, E. J. & Wilson, I. D. Liquid chromatography-mass spectrometry based global metabolite profiling: a review. Anal. Chim. Acta 711, 7–16 (2012).
Cajka, T. & Fiehn, O. Toward merging untargeted and targeted methods in mass spectrometry-based metabolomics and lipidomics. Anal. Chem. 88, 524–545 (2016).
Hubbard, T. D., Murray, I. A. & Perdew, G. H. Indole and tryptophan metabolism: endogenous and dietary routes to Ah receptor activation. Drug Metab. Dispos. 43, 1522–1535 (2015).
Ganguli, K. & Walker, W. A. Treatment of necrotizing enterocolitis with probiotics. Gastroenterol. Clin. N. Am. 41, 733–746 (2012).
Olsen, R., Greisen, G., Schrøder, M. & Brok, J. Prophylactic probiotics for preterm infants: a systematic review and meta-analysis of observational studies. Neonatology 109, 105–112 (2016).
Henry, M. C. W. & Moss, R. L. Necrotizing enterocolitis. Annu. Rev. Med. 60, 111–124 (2009).
Good, M. et al. Breast milk protects against the development of necrotizing enterocolitis through inhibition of Toll-like receptor 4 in the intestinal epithelium via activation of the epidermal growth factor receptor. Mucosal. Immunol. 8, 1166–1179 (2015).
Roager, H. M. & Licht, T. R. Microbial tryptophan catabolites in health and disease. Nat. Commun. 9, 3294–3300 (2018).
Vogel, C. F. et al. RelB, a new partner of aryl hydrocarbon receptor-mediated transcription. Mol. Endocrinol. 21, 2941–2955 (2007).
Ehrlich, A. M. et al. Bifidobacterium grown on human milk oligosaccharides produce tryptophan metabolite indole-3-lactic acid that significantly decreases inflammation in intestinal cells in vitro. FASEB J. 32, Ib359 (2018).
Aoki, R., Aoki-Yoshida, A., Suzuki, C. & Takayama, Y. Indole-3-pyruvic acid, an aryl hydrocarbon receptor activator, suppresses experimental colitis in mice. J. Immunol. 201, 3683–3693 (2018).
Aragozzini, F., Ferrari, A., Pacini, N. & Gualandris, R. Indole-3-lactic acid as a tryptophan metabolite produced by Bifidobacterium spp. Appl. Environ. Microbiol. 38, 544–546 (1979).
Laursen, M., Bahl, M., Michaelsen, K. & Licht, T. First foods and gut microbes. Front. Microbiol. 8, 356 (2017).
Nikolaus, S. et al. Increased tryptophan metabolism is associated with activity of inflammatory bowel diseases. Gastroenterology 153, 1504–1516 (2017).
Rothhammer, V. Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat. Med. 22, 586–597 (2016).
Acknowledgements
This study was supported by the following grants: NIDDK (P01-DK033506) “Barrier function of the GI tract in health and disease” PI W.A.W.; Family Larsson-Rosenquist Foundation “The impact of breastmilk microbiome and protective factors on development of human intestinal disease” PI W.A.W.; Beth Israel/Deaconess Medical Center (Award #01027741) “Impact of breastmilk in premature intestinal colonization” PI W.A.W.
Author information
Authors and Affiliations
Contributions
D.M. oversaw all experiments; E.S., P.C. and E.S. analyzed the B. infantis fractions and identified ILA; K.G., K.D. and W.Z. contributed to ELISA and LDH assay analysis, cell line maintenance, and mouse experiments; W.A.W. conceived of the research plan and analyzed experimental data.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Meng, D., Sommella, E., Salviati, E. et al. Indole-3-lactic acid, a metabolite of tryptophan, secreted by Bifidobacterium longum subspecies infantis is anti-inflammatory in the immature intestine. Pediatr Res 88, 209–217 (2020). https://doi.org/10.1038/s41390-019-0740-x
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41390-019-0740-x
This article is cited by
-
Lactobacilli-based postbiotic differentially affects chicken macrophage-like HD11 cells depending on stimulatory lipopolysaccharide dosage
BMC Veterinary Research (2025)
-
Heightened innate immunity may trigger chronic inflammation, fatigue and post-exertional malaise in ME/CFS
npj Metabolic Health and Disease (2025)
-
Bifidobacterium deficit in United States infants drives prevalent gut dysbiosis
Communications Biology (2025)
-
Synergism between TLR4 and B. infantis in the development of the premature intestine
Pediatric Research (2025)
-
Bifidobacterium longum subsp infantis (EVC001) is associated with reduced incidence of necrotizing enterocolitis stage ≥2 and bloody stools in premature babies
Journal of Perinatology (2025)