Abstract
Background
The rate of accrual of muscle mass in neonates has not been assessed. We describe the D3-creatine (D3Cr) dilution method, a noninvasive assessment of muscle mass in neonates.
Methods
A total of 76 neonates >26-week-old corrected gestational age were enrolled and measured at 2-week intervals while admitted to a neonatal intensive care unit (NICU). Additional measures at 6 and 12–20 months after initial measurement were obtained if available. An enteral dose of 2 mg D3Cr in 0.5 mL 20% 2H2O was used to determine muscle mass and total body water (TBW).
Results
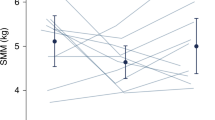
Muscle mass by the D3Cr method was strongly associated with TBW and body weight (r = 0.9272, p < 0.0001 and r = 0.9435, p < 0.0001 for all time points and r = 0.6661, p < 0.0001 and r = 0.8634, p < 0.0001, respectively, while in the NICU). Change in muscle mass vs. change in body weight, TBW, and length were also strongly correlated.
Conclusions
The D3Cr dilution method provides a noninvasive assessment of muscle mass accrual in neonates, which has not been previously possible and may be an important new tool for the evaluation of nutritional status and normal growth patterns.
Impact
-
We describe a noninvasive method for the measurement of skeletal muscle mass neonates.
-
At the present time, there is no direct measurement of muscle mass in infants available.
-
The D3Cr dilution method is a direct and noninvasive measurement of muscle mass.
-
Using a single enteral dose of D3Cr in 2H2O followed by urine and saliva samples, rapid and substantial accrual of muscle mass and TBW is assessed.
-
Assessment of muscle mass accrual in premature infants may be a strong indicator of nutritional status.
-
Change in muscle mass is strongly related to change in weight and TBW.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Wolfe, R. R. The underappreciated role of muscle in health and disease. Am. J. Clin. Nutr. 84, 475–482 (2006).
Saggese, G., Fanos, M. & Simi, F. SGA children: auxological and metabolic outcomes—the role of GH treatment. J. Matern. Fetal Neonatal Med. 26, 64–67 (2013).
Stimpson, S. A. et al. Longitudinal changes in total body creatine pool size and skeletal muscle mass using the D-creatine dilution method. J. Cachexia Sarcopenia Muscle 4, 217–223 (2013).
Stimpson, S. A. et al. Total-body creatine pool size and skeletal muscle mass determination by creatine-(methyl-D3) dilution in rats. J. Appl. Physiol. 112, 1940–1948 (2012).
Clark, R. V. et al. Total body skeletal muscle mass: estimation by creatine (methyl-d3) dilution in humans. J. Appl. Physiol. 116, 1605–1613 (2014).
Cawthon, P. M. et al. Strong relation between muscle mass determined by D3-creatine dilution, physical performance, and incidence of falls and mobility limitations in a prospective cohort of older men. J. Gerontol. A 74, 844–852 (2019).
Cawthon, P. M. et al. Muscle mass assessed by D3-creatine dilution method and incident self-reported disability and mortality in a prospective observational study of community dwelling older men. J. Gerontol. A, glaa111 (2020). https://doi.org/10.1093/gerona/glaa111 [online ahead of print].
Ehrenkranz, R. A. et al. Growth in the neonatal intensive care unit influences neurodevelopmental and growth outcomes of extremely low birth weight infants. Pediatrics 117, 1253–1261 (2006).
Horbar, J. D. et al. Weight growth velocity and postnatal growth failure in infants 501 to 1500 grams: 2000–2013. Pediatrics 136, e84–e92 (2015).
Griffin, I. J., Tancredi, D. J., Bertino, E., Lee, H. C. & Profit, J. Postnatal growth failure in very low birthweight infants born between 2005 and 2012. Arch. Dis. Child Fetal Neonatal Ed. 101, F50–F55 (2016).
Barker, D. J. The malnourished baby and infant. Br. Med. Bull. 60, 69–88 (2001).
Godfrey, K. M. & Barker, D. J. Fetal programming and adult health. Public Health Nutr. 4, 611–624 (2001).
Hediger, M. L. et al. Muscularity and fatness of infants and young children born small- or large-for-gestational-age. Pediatrics 102, E60 (1998).
Faith, M. S. et al. Evidence for independent genetic influences on fat mass and body mass index in a pediatric twin sample. Pediatrics 104, 61–67 (1999).
Nunez, C. et al. Body composition in children and adults by air displacement plethysmography. Eur. J. Clin. Nutr. 53, 382–387 (1999).
Shankaran, M. et al. Dilution of oral D3-creatine to measure creatine pool size and estimate skeletal muscle mass: development of a correction algorithm. J. Cachexia Sarcopenia Muscle 9, 540–546 (2018).
Schoeller, D. A. et al. Total body water measurement in humans with 18O and 2H labeled water. Am. J. Clin. Nutr. 33, 2686–2693 (1980).
Ellis, K. J. Evaluation of body composition in neonates and infants. Semin. Fetal Neonatal Med. 12, 87–91 (2007).
Hartnoll, G., Betremieux, P. & Modi, N. Body water content of extremely preterm infants at birth. Arch. Dis. Child Fetal Neonatal Ed. 83, F56–F59 (2000).
Orwoll, E. S. et al. The importance of muscle versus fat mass in sarcopenic obesity: a re-evaluation using D3-creatine muscle mass versus DXA lean mass measurements. J. Gerontol. A 75, 1362–1368 (2020).
Scheurer, J. M. et al. Body composition changes from infancy to 4 years and associations with early childhood cognition in preterm and full-term children. Neonatology 114, 169–176 (2018).
Pfister, K. M. et al. Early body composition changes are associated with neurodevelopmental and metabolic outcomes at 4 years of age in very preterm infants. Pediatr. Res. 84, 713–718 (2018).
Belfort, M. B., Gillman, M. W., Buka, S. L., Casey, P. H. & McCormick, M. C. Preterm infant linear growth and adiposity gain: trade-offs for later weight status and intelligence quotient. J. Pediatr. 163, 1564–1569 e1562 (2013).
Ramel, S. E. et al. The relationship of poor linear growth velocity with neonatal illness and two-year neurodevelopment in preterm infants. Neonatology 102, 19–24 (2012).
Wood, A. J., Raynes-Greenow, C. H., Carberry, A. E. & Jeffery, H. E. Neonatal length inaccuracies in clinical practice and related percentile discrepancies detected by a simple length-board. J. Paediatr. Child Health 49, 199–203 (2013).
Barker, D. J. The developmental origins of well-being. Philos. Trans. R. Soc. Lond. Ser. B 359, 1359–1366 (2004).
Acknowledgements
We thank Jeanette Asselin, M.S., at the University of California San Francisco Benioff Children’s Hospital at Oakland for early recruitment and enrollment of infants in this study. All phases of this study were supported by the Bill and Melinda Gates Foundation.
Author information
Authors and Affiliations
Contributions
W.J.E., M.H., and M.S. conceptualized and designed the study, interpreted the data, drafted the initial manuscript, and reviewed and revised the manuscript. B.S. and F.I. implemented the study, acquired the data, interpreted the data, and reviewed and revised the manuscript. E.N., K.G., and G.C. acquired the data, interpreted the analysis, and reviewed and revised the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
W.J.E., M.H., and M.S. were employed by Kinemed, Inc. for a period of time during the conduct of this project. W.J.E. and M.H. are listed as co-inventors on the granted patents for the D3-creatine dilution method, but do not own or derive any income from the intellectual property.
Consent statement
The study was approved by the Oregon Health & Sciences University Medical School and University of California San Francisco Benioff Children’s Hospital at Oakland Institutional Review Boards. Parents provided consent for all infants enrolled in the study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Evans, W.J., Scottoline, B., Imam, F. et al. D3-creatine dilution for the noninvasive measurement of skeletal muscle mass in premature infants. Pediatr Res 89, 1508–1514 (2021). https://doi.org/10.1038/s41390-020-01122-w
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-020-01122-w
This article is cited by
-
Association of body composition measures to muscle strength using DXA, D3Cr, and BIA in collegiate athletes
Scientific Reports (2025)
-
Skeletal muscle mass can be estimated by creatine (methyl‐d3) dilution and is correlated with fat-free mass in active young males
European Journal of Clinical Nutrition (2023)
-
Estimation of skeletal muscle mass in 4-year-old children using the D3-creatine dilution method
Pediatric Research (2023)
-
D3Creatine Dilution as a Direct, Non-invasive and Accurate Measurement of Muscle Mass for Aging Research
Calcified Tissue International (2023)
-
Adapting MultiPLe behavior Interventions that eFfectively Improve (AMPLIFI) cancer survivor health: program project protocols for remote lifestyle intervention and assessment in 3 inter-related randomized controlled trials among survivors of obesity-related cancers
BMC Cancer (2022)