Abstract
Background
Identifying preterm infants at risk for mortality or major morbidity traditionally relies on gestational age, birth weight, and other clinical characteristics that offer underwhelming utility. We sought to determine whether a newborn metabolic vulnerability profile at birth can be used to evaluate risk for neonatal mortality and major morbidity in preterm infants.
Methods
This was a population-based retrospective cohort study of preterm infants born between 2005 and 2011 in California. We created a newborn metabolic vulnerability profile wherein maternal/infant characteristics along with routine newborn screening metabolites were evaluated for their association with neonatal mortality or major morbidity.
Results
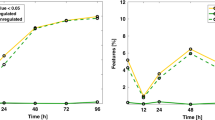
Nine thousand six hundred and thirty-nine (9.2%) preterm infants experienced mortality or at least one complication. Six characteristics and 19 metabolites were included in the final metabolic vulnerability model. The model demonstrated exceptional performance for the composite outcome of mortality or any major morbidity (AUC 0.923 (95% CI: 0.917–0.929). Performance was maintained across mortality and morbidity subgroups (AUCs 0.893–0.979).
Conclusions
Metabolites measured as part of routine newborn screening can be used to create a metabolic vulnerability profile. These findings lay the foundation for targeted clinical monitoring and further investigation of biological pathways that may increase the risk of neonatal death or major complications in infants born preterm.
Impact
-
We built a newborn metabolic vulnerability profile that could identify preterm infants at risk for major morbidity and mortality.
-
Identifying high-risk infants by this method is novel to the field and outperforms models currently in use that rely primarily on infant characteristics.
-
Utilizing the newborn metabolic vulnerability profile for precision clinical monitoring and targeted investigation of etiologic pathways could lead to reductions in the incidence and severity of major morbidities associated with preterm birth.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Martin, J. A., Hamilton, B. E., Osterman, M. J. K., Driscoll, A. K. & Drake, P. Births: final data for 2016. Natl Vital Stat. Rep. 67, 1–55 (2018).
Chawanpaiboon, S. et al. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Glob. Health 7, e37–e46 (2018).
Liu, L. et al. Global, regional, and national causes of under-5 mortality in 2000-15: an updated systematic analysis with implications for the sustainable development goals. Lancet 388, 3027–3035 (2016).
Liu, L. et al. Global, regional, and national causes of child mortality in 2000-13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet 385, 430–440 (2015).
UN Inter-Agency Group. Levels & Trends in Child Mortality: Report 2017 (UN Inter-Agency Group for Child Mortality Estimation, New York, 2017).
Benitz, W. E. & Committee on Fetus and Newborn. Patent ductus arteriosus in preterm infants. Pediatrics 137, e20153730 (2016).
Platt, M. J. Outcomes in preterm infants. Public Health 128, 399–403 (2014).
Huang, J. et al. Association between perinatal hypoxic-ischemia and periventricular leukomalacia in preterm infants: a systematic review and meta-analysis. PLoS ONE 12, e0184993 (2017).
Wang, L. W., Lin, Y. C., Wang, S. T. & Huang, C. C. Identifying risk factors shared by bronchopulmonary dysplasia, severe retinopathy, and cystic periventricular leukomalacia in very preterm infants for targeted intervention. Neonatology 114, 17–24 (2018).
Saigal, S. & Doyle, L. W. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet 371, 261–269 (2008).
Mwaniki, M. K., Atieno, M., Lawn, J. E. & Newton, C. R. Long-term neurodevelopmental outcomes after intrauterine and neonatal insults: a systematic review. Lancet 379, 445–452 (2012).
Purisch, S. E. & Gyamfi-Bannerman, C. Epidemiology of preterm birth. Semin. Perinatol. 41, 387–391 (2017).
Ancel, P. Y. et al. Survival and morbidity of preterm children born at 22 through 34 weeks’ gestation in France in 2011: results of the EPIPAGE-2 cohort study. JAMA Pediatr. 169, 230–238 (2015).
Anderson, J. G. et al. Survival and major morbidity of extremely preterm infants: a population-based study. Pediatrics 138, e20154434 (2016).
Hintz, S. R. et al. Changes in mortality and morbidities among infants born at less than 25 weeks during the post-surfactant era. Arch. Dis. Child. Fetal Neonatal Ed. 90, F128–F133 (2005).
Kyser, K. L., Morriss, F. H. Jr., Bell, E. F., Klein, J. M. & Dagle, J. M. Improving survival of extremely preterm infants born between 22 and 25 weeks of gestation. Obstet. Gynecol. 119, 795–800 (2012).
Rysavy, M. A. et al. Between-hospital variation in treatment and outcomes in extremely preterm infants. N. Engl. J. Med. 372, 1801–1811 (2015).
Younge, N. et al. Survival and neurodevelopmental outcomes among periviable infants. N. Engl. J. Med. 376, 617–628 (2017).
Tyson, J. E. et al. Intensive care for extreme prematurity-moving beyond gestational age. N. Engl. J. Med. 358, 1672–1681 (2008).
Lawn, J. E. et al. Every newborn: progress, priorities, and potential beyond survival. Lancet 384, 189–205 (2014).
Bennett, M. J. Newborn screening for metabolic diseases: saving children’s lives and improving outcomes. Clin. Biochem. 47, 693–694 (2014).
Ryckman, K. K. et al. Association of amino acids with common complications of prematurity. Pediatr. Res. 73, 700–705 (2013).
Fell, D. B. et al. Using newborn screening analytes to identify cases of neonatal sepsis. Sci. Rep. 7, 18020 (2017).
McCarthy, M. E. et al. Newborn metabolic profile associated with hyperbilirubinemia with and without kernicterus. Clin. Transl. Sci. 12, 28–38 (2018).
Oltman, S. P. et al. Initial metabolic profiles are associated with 7-day survival among infants born at 22-25 weeks of gestation. J. Pediatr. 198, 194.e3–200.e3 (2018).
Steurer, M. A. et al. Altered metabolites in newborns with persistent pulmonary hypertension. Pediatr. Res. 84, 272–278 (2018).
Sylvester, K. G. et al. Acylcarnitine profiles reflect metabolic vulnerability for necrotizing enterocolitis in newborns born premature. J. Pediatr. 181, 80.e1–85.e1 (2016).
Feuchtbaum, L., Carter, J., Dowray, S., Currier, R. J. & Lorey, F. Birth prevalence of disorders detectable through newborn screening by race/ethnicity. Genet. Med. 14, 937–945 (2012).
Jelliffe-Pawlowski, L. L., Norton, M. E., Baer, R. J., Santos, N. & Rutherford, G. W. Gestational dating by metabolic profile at birth: a California cohort study. Am. J. Obstet. Gynecol. 214, 511.e511–511.e513 (2016).
Talge, N. M., Mudd, L. M., Sikorskii, A. & Basso, O. United States birth weight reference corrected for implausible gestational age estimates. Pediatrics 133, 844–853 (2014).
Kotelchuck, M. The Adequacy of Prenatal Care Utilization Index: its US distribution and association with low birthweight. Am. J. Public Health 84, 1486–1489 (1994).
Kotelchuck, M. An evaluation of the Kessner Adequacy of Prenatal Care Index and a proposed Adequacy of Prenatal Care Utilization Index. Am. J. Public Health 84, 1414–1420 (1994).
Lin, H. J. et al. Extremely high phenylalanine levels in a newborn on parenteral nutrition: phenylketonuria in the neonatal intensive care unit. J. Perinatol. 31, 507–510 (2011).
Morris, M. et al. Reduction in newborn screening metabolic false-positive results following a new collection protocol. Genet. Med. 16, 477–483 (2014).
Ryckman, K. K., Berberich, S. L., Shchelochkov, O. A., Cook, D. E. & Murray, J. C. Clinical and environmental influences on metabolic biomarkers collected for newborn screening. Clin. Biochem. 46, 133–138 (2013).
Natarajan, G. & Shankaran, S. Short- and long-term outcomes of moderate and late preterm infants. Am. J. Perinatol. 33, 305–317 (2016).
Patel, R. M. Short- and long-term outcomes for extremely preterm infants. Am. J. Perinatol. 33, 318–328 (2016).
Baer, R. J. et al. Population-based risks of mortality and preterm morbidity by gestational age and birth weight. J. Perinatol. 36, 1008–1013 (2016).
Horbar, J. D. et al. Mortality and neonatal morbidity among infants 501 to 1500 grams from 2000 to 2009. Pediatrics 129, 1019–1026 (2012).
Donovan, B. M. et al. Association of newborn screening metabolites with risk of wheezing in childhood. Pediatr. Res. 84, 619–624 (2018).
Costeloe, K. L. et al. Short term outcomes after extreme preterm birth in England: comparison of two birth cohorts in 1995 and 2006 (the EPICure studies). BMJ 345, e7976 (2012).
Jelliffe-Pawlowski, L. L., Norton, M. E., Baer, R. J., Santos, N. & Rutherford, G. W. Gestational dating by metabolic profile at birth: a California cohort study. Am. J. Obstet. Gynecol. 214, 511.e1–511.e13 (2015).
Ryckman, K. K., Berberich, S. L. & Dagle, J. M. Predicting gestational age using neonatal metabolic markers. Am. J. Obstet. Gynecol. 214, 515.e1–515.e13 (2016).
Wilson, K. et al. Accurate prediction of gestational age using newborn screening analyte data. Am. J. Obstet. Gynecol. 214, 513.e1–513.e9 (2016).
Atzori, L. et al. 1H NMR-based metabolomic analysis of urine from preterm and term neonates. Front. Biosci. (Elite Ed.) 3, 1005–1012 (2011).
Wilson, K. et al. Metabolomics of prematurity: analysis of patterns of amino acids, enzymes, and endocrine markers by categories of gestational age. Pediatr. Res. 75, 367–373 (2014).
Clark, R. H., Kelleher, A. S., Chace, D. H. & Spitzer, A. R. Gestational age and age at sampling influence metabolic profiles in premature infants. Pediatrics 134, e37–e46 (2014).
Ryckman, K. K., Spracklen, C. N., Dagle, J. M. & Murray, J. C. Maternal factors and complications of preterm birth associated with neonatal thyroid stimulating hormone. J. Pediatr. Endocrinol. Metab. 27, 929–938 (2014).
Kantor, M. J., Leef, K. H., Bartoshesky, L., Getchell, J. & Paul, D. A. Admission thyroid evaluation in very-low-birth-weight infants: association with death and severe intraventricular hemorrhage. Thyroid 13, 965–969 (2003).
Redding, R. A. & Pereira, C. Thyroid function in respiratory distress syndrome (RDS) of the newborn. Pediatrics 54, 423–428 (1974).
Hitchcock, K. R. Lung development and the pulmonary surfactant system: hormonal ifluences. Anat. Rec. 198, 13–34 (1980).
Cuestas, R. A., Lindall, A. & Engel, R. R. Low thyroid hormones and respiratory-distress syndrome of the newborn. Studies on cord blood. N. Engl. J. Med. 295, 297–302 (1976).
Panicker, V. Genetics of thyroid function and disease. Clin. Biochem. Rev. 32, 165–175 (2011).
Simpson, J. et al. Serum thyroid hormones in preterm infants and relationships to indices of severity of intercurrent illness. J. Clin. Endocrinol. Metab. 90, 1271–1279 (2005).
Thony, B., Auerbach, G. & Blau, N. Tetrahydrobiopterin biosynthesis, regeneration and functions. Biochem. J. 347(Pt 1), 1–16 (2000).
Ploder, M. et al. Serum phenylalanine in patients post trauma and with sepsis correlate to neopterin concentrations. Amino Acids 35, 303–307 (2008).
Gitto, E., Pellegrino, S., D’Arrigo, S., Barberi, I. & Reiter, R. J. Oxidative stress in resuscitation and in ventilation of newborns. Eur. Respir. J. 34, 1461–1469 (2009).
Tataranno, M. L., Perrone, S. & Buonocore, G. Plasma biomarkers of oxidative stress in neonatal brain injury. Clin. Perinatol. 42, 529–539 (2015).
Vento, M. et al. Preterm resuscitation with low oxygen causes less oxidative stress, inflammation, and chronic lung disease. Pediatrics 124, e439–e449 (2009).
Becker, R. M. et al. Reduced serum amino acid concentrations in infants with necrotizing enterocolitis. J. Pediatr. 137, 785–793 (2000).
Pearson, D. L. et al. Neonatal pulmonary hypertension-urea-cycle intermediates, nitric oxide production, and carbamoyl-phosphate synthetase function. N. Engl. J. Med. 344, 1832–1838 (2001).
Celik, I. H., Demirel, G., Canpolat, F. E. & Dilmen, U. Reduced plasma citrulline levels in low birth weight infants with necrotizing enterocolitis. J. Clin. Lab. Anal. 27, 328–332 (2013).
Hediger, N., Landolt, M. A., Diez-Fernandez, C., Huemer, M. & Haberle, J. The impact of ammonia levels and dialysis on outcome in 202 patients with neonatal onset urea cycle disorders. J. Inherit. Metab. Dis. 41, 689–698 (2018).
Ruder, J., Legacy, J., Russo, G. & Davis, R. Neonatal citrullinemia: novel, reversible neuroimaging findings correlated with ammonia level changes. Pediatr. Neurol. 51, 553–556 (2014).
Longo, N., Frigeni, M. & Pasquali, M. Carnitine transport and fatty acid oxidation. Biochim. Biophys. Acta 1863, 2422–2435 (2016).
Sauer, S. W., Okun, J. G., Hoffmann, G. F., Koelker, S. & Morath, M. A. Impact of short- and medium-chain organic acids, acylcarnitines, and acyl-CoAs on mitochondrial energy metabolism. Biochim. Biophys. Acta 1777, 1276–1282 (2008).
Deshmukh, M. & Patole, S. Antenatal corticosteroids for neonates born before 25 weeks-a systematic review and meta-analysis. PLoS ONE 12, e0176090 (2017).
Travers, C. P. et al. Exposure to any antenatal corticosteroids and outcomes in preterm infants by gestational age: prospective cohort study. BMJ 356, j1039 (2017).
Travers, C. P. et al. Mortality and pulmonary outcomes of extremely preterm infants exposed to antenatal corticosteroids. Am. J. Obstet. Gynecol. 218, 130.e1–130.e13 (2018).
Acknowledgements
Data from the California Prenatal and Newborn Screening Programs were obtained through the California Biobank Program (Screening Information System request no. 476). Data were obtained with an agreement that the California Department of Public Health is not responsible for the results or conclusions drawn by the authors of this publication. This work was supported by the California Preterm Birth Initiative within the University of California, San Francisco (UCSF).
Author information
Authors and Affiliations
Contributions
S.P.O., E.E.R., R.J.B., K.K.R., and L.L.J.-P. contributed to the conception and design, acquisition of data, or analysis and interpretation of data. All authors contributed to drafting the manuscript or revising it critically for important intellectual content All authors also gave final approval of the version to be published.
Corresponding author
Ethics declarations
Competing interests
No authors have any financial ties to products in the study or potential/perceived conflicts of interest.
Consent statement
The State of California granted a waiver of consent for this study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Oltman, S.P., Rogers, E.E., Baer, R.J. et al. Newborn metabolic vulnerability profile identifies preterm infants at risk for mortality and morbidity. Pediatr Res 89, 1405–1413 (2021). https://doi.org/10.1038/s41390-020-01148-0
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41390-020-01148-0
This article is cited by
-
Newborn screen metabolic panels reflect the impact of common disorders of pregnancy
Pediatric Research (2022)