Abstract
Background
The mechanism of bilirubin neurotoxicity is poorly understood. We hypothesize that bilirubin inhibits the function of lipid rafts (LR), microdomains of the plasma membrane critical for signal transduction. To test this hypothesis, we measured the effect of free bilirubin (Bf) between 7.6 and 122.5 nM on LR-dependent functions of L1 cell adhesion molecule (L1).
Methods
Cerebellar granule neurons (CGN) were plated on poly-l-lysine overnight, and neurite length was determined after 1 h treatment with L1 alone or L1 and bilirubin. L1 activation of ERK1/2 was measured in CGN in the presence or absence of bilirubin. The effect of bilirubin on L1 distribution in LR was quantitated, and the localization of bilirubin to LR was determined.
Results
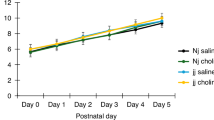
The addition of bilirubin to CGN treated with L1 significantly decreased neurite length compared to L1 alone. L1 activation of ERK1/2 was inhibited by bilirubin. Bilirubin redistributed L1 into LR. Bilirubin was associated only with LR-containing fractions of a sucrose density gradient.
Conclusion
Bf significantly inhibits LR-dependent functions of L1 and are found only associated with LR, suggesting one mechanism by which bilirubin may exert neurotoxicity is through the dysfunction of protein–LR interactions.
Impact
-
This article establishes lipid rafts as a target for the neurotoxic effects of bilirubin.
-
This article provides clear evidence toward establishing one mechanism of bilirubin neurotoxicity, where little is understood.
-
This article paves the way for future investigation into lipid raft dependent functions, and its role in neurodevelopmental outcome.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Shapiro, S. M. & Nakamura, H. Bilirubin and the auditory system. J. Perinatol. 21(Suppl. 1), S52–S55 (2001). Discussion S59–S62.
Arnold, C., Pedroza, C. & Tyson, J. E. Phototherapy in ELBW newborns: does it work? Is it safe? The evidence from randomized clinical trials. Semin. Perinatol. 38, 452–464 (2014).
Xoinis, K. et al. Extremely low birth weight infants are at high risk for auditory neuropathy. J. Perinatol. 27, 718–723 (2007).
Biran, V., Verney, C. & Ferriero, D. M. Perinatal cerebellar injury in human and animal models. Neurol. Res. Int. 2012, 858929 (2012).
Bhutani, V. K., Wong, R. J. & Stevenson, D. K. Hyperbilirubinemia in preterm neonates. Clin. Perinatol. 43, 215–232 (2016).
Amin, S. B. & Lamola, A. A. Newborn jaundice technologies: unbound bilirubin and bilirubin binding capacity in neonates. Semin. Perinatol. 35, 134–140 (2011).
Malik, S. G., Irwanto, K. A., Ostrow, J. D. & Tiribelli, C. Effect of bilirubin on cytochrome c oxidase activity of mitochondria from mouse brain and liver. BMC Res. Notes 3, 162 (2010).
Ahlfors, C. E. Predicting bilirubin neurotoxicity in jaundiced newborns. Curr. Opin. Pediatr. 22, 129–133 (2010).
Shapiro, S. M. Definition of the clinical spectrum of kernicterus and bilirubin-induced neurologic dysfunction (BIND). J. Perinatol. 25, 54–59 (2005).
Ostrow, J. D., Pascolo, L. & Tiribelli, C. Reassessment of the unbound concentrations of unconjugated bilirubin in relation to neurotoxicity in vitro. Pediatr. Res. 54, 98–104 (2003).
Danbolt, C. et al. In vitro binding of [3H]bilirubin to neurons in rat brain sections. Biol. Neonate 63, 35–39 (1993).
Ostrow, J. D., Pascolo, L., Brites, D. & Tiribelli, C. Molecular basis of bilirubin-induced neurotoxicity. Trends Mol. Med. 10, 65–70 (2004).
Mukerjee, P. & Ostrow, J. D. Interactions of unconjugated bilirubin with vesicles, cyclodextrins and micelles: new modeling and the role of high pKa values. BMC Biochem. 11, 15 (2010).
Simons, K. & Gerl, M. J. Revitalizing membrane rafts: new tools and insights. Nat. Rev. Cell Biol. 11, 688–699 (2010).
Patel, H. H., Murray, F. & Insel, P. A. Caveolae as organizers of pharmacologically relevant signal transduction molecules. Annu. Rev. Pharm. Toxicol. 48, 359–391 (2008).
Epshtein, Y. et al. Identification of a C-terminus domain critical for the sensitivity of Kir2.1 to cholesterol. Proc. Natl Acad. Sci. USA 106, 8055–8060 (2009).
Fantini, J. & Barrantes, F. J. Sphingolipid/cholesterol regulation of neurotransmitter receptor conformation and function. Biochim. Biophys. Acta 1788, 2345–2361 (2009).
Simons, K. & Ikonen, E. Functional rafts in cell membranes. Nature 387, 569–572 (1997).
Dart, C. Lipid microdomains and the regulation of ion channel function. J. Physiol. 588, 3169–3178 (2010).
Simons, K. & Ehehalt, R. Cholesterol, lipid rafts, and disease. J. Clin. Invest. 110, 597–603 (2002).
Owen, D. M., Magenau, A., Williamson, D. & Gaus, K. The lipid raft hypothesis revisited-new insights on raft composition and function from super-resolution fluorescence microscopy. Bioessays 34, 739–747 (2012).
Korade, Z. & Kenworthy, A. K. Lipid rafts, cholesterol, and the brain. Neuropharmacology 55, 1265–1273 (2008).
Pike, L. J. The challenge of lipid rafts. J. Lipid Res. 50(Suppl.), S323–S328 (2009).
Nakai, Y. & Kamiguchi, H. Migration of nerve growth cones requires detergent-resistant membranes in a spatially defined and substrate-dependent manner. J. Cell Biol. 159, 1097–1108 (2002).
Tang, N. et al. Ethanol causes the redistribution of L1 cell adhesion molecule in lipid rafts. J. Neurochem. 119, 859–867 (2011).
Kamiguchi, H. & Lemmon, V. Recycling of the cell adhesion molecule L1 in axonal growth cones. J. Neurosci. 20, 3676–3686 (2000).
Schmid, R. S., Pruitt, W. M. & Maness, P. F. A. MAP kinase-signaling pathway mediates neurite outgrowth on L1 and requires Src-dependent endocytosis. J. Neurosci. 20, 4177–4188 (2000).
Yeaney, N. K. et al. Ethanol inhibits L1 cell adhesion molecule tyrosine phosphorylation and dephosphorylation and activation of pp60(src). J. Neurochem. 110, 779–790 (2009).
Watanabe, H. et al. Phospholipase D2 functions as a downstream signaling molecule of MAP kinase pathway in L1-stimulated neurite outgrowth of cerebellar granule neurons. J. Neurochem. 89, 142–151 (2004).
Milstone, A. M. et al. Chlorhexidine inhibits L1 cell adhesion molecule-mediated neurite outgrowth in vitro. Pediatr. Res. 75, 8–13 (2014).
Tang, N. et al. Choline partially prevents the impact of ethanol on the lipid raft dependent functions of l1 cell adhesion molecule. Alcohol Clin. Exp. Res. 38, 2722–2730 (2014).
Gonzalez-Reyes, S. et al. Neuroprotective role of heme-oxygenase 1 against iodoacetate-induced toxicity in rat cerebellar granule neurons: Role of bilirubin. Free Radic. Res. 43, 214–223 (2009).
Ahlfors, C. E. Measurement of plasma unbound unconjugated bilirubin. Anal. Biochem. 279, 130–135 (2000).
Shapiro, S. M., Sombati, S., Geiger, A. & Rice, A. C. NMDA channel antagonist MK-801 does not protect against bilirubin neurotoxicity. Neonatology 92, 248–257 (2007).
Bearer, C. F., Swick, A. R., O’Riordan, M. A. & Cheng, G. Ethanol inhibits L1-mediated neurite outgrowth in postnatal rat cerebellar granule cells. J. Biol. Chem. 274, 13264–13270 (1999).
Tang, N. et al. Ethanol inhibits L1 cell adhesion molecule activation of mitogen-activated protein kinases. J. Neurochem. 96, 1480–1490 (2006).
Mustafa, M. G. & King, T. E. Binding of bilirubin with lipid. A possible mechanism of its toxic reactions in mitochondria. J. Biol. Chem. 245, 1084–1089 (1970).
Diamond, I. & Schmid, R. Oxidative phosphorylation in experimental bilirubin encephalopathy. Science 155, 1288–1289 (1967).
Daood, M. J., Hoyson, M. & Watchko, J. F. Lipid peroxidation is not the primary mechanism of bilirubin-induced neurologic dysfunction in jaundiced Gunn rat pups. Pediatr. Res. 72, 455–459 (2012).
Nakai, Y. & Kamiguchi, H. Migration of nerve growth cones requires detergent-resistant membranes in a spatially defined and substrate-dependent manner. J. Cell Biol. 159, 1097–1108 (2002).
Šmíd, V. et al. Heme oxygenase-1 may affect cell signalling via modulation of ganglioside composition. Oxid. Med. Cell Longev. 2018, 3845027 (2018).
Mancuso, C. et al. Bilirubin as an endogenous modulator of neurotrophin redox signaling. J. Neurosci. Res. 86, 2235–2249 (2008).
Fernandes, A. et al. MAPKs are key players in mediating cytokine release and cell death induced by unconjugated bilirubin in cultured rat cortical astrocytes. Eur. J. Neurosci. 25, 1058–1068 (2007).
Peyton, K. J. et al. Bilirubin inhibits neointima formation and vascular smooth muscle cell proliferation and migration. Front. Pharmacol. 3, 48 (2012).
Öllinger, R. et al. Bilirubin inhibits tumor cell growth via activation of ERK. Cell Cycle 6, 3078–3085 (2007).
Lee, Y. K. et al. The significance of measurement of serum unbound bilirubin concentrations in high-risk infants. Pediatr. Int. 51, 795–799 (2009).
van der Schoor, L. W. et al. Unconjugated free bilirubin in preterm infants. Early Hum. Dev. 106–107, 25–32 (2017).
Hulzebos, C. V. et al. The bilirubin albumin ratio in the management of hyperbilirubinemia in preterm infants to improve neurodevelopmental outcome: a randomized controlled trial-BARTrial. PLoS ONE 9, e99466 (2014).
Amin, S. B. & Wang, H. Bilirubin albumin binding and unbound unconjugated hyperbilirubinemia in premature infants. J. Pediatr. 192, 47–52 (2018).
Bearer, C. F. et al. Choline ameliorates deficits in balance caused by acute neonatal ethanol exposure. Cerebellum 14, 413–420 (2015).
Davis, N. L. et al. Choline ameliorates ethanol induced alterations in tyrosine phosphorylation and distribution in detergent-resistant membrane microdomains of L1 cell adhesion molecule in vivo. Birth Defects Res. 112, 480–489 (2020).
Monk, B. R., Leslie, F. M. & Thomas, J. D. The effects of perinatal choline supplementation on hippocampal cholinergic development in rats exposed to alcohol during the brain growth spurt. Hippocampus 22, 1750–1757 (2012).
Thomas, J. D., Idrus, N. M., Monk, B. R. & Dominguez, H. D. Prenatal choline supplementation mitigates behavioral alterations associated with prenatal alcohol exposure in rats. Birth Defects Res. A Clin. Mol. Teratol. 88, 827–837 (2010).
Waddell, J. & Mooney, S. M. Choline and working memory training improve cognitive deficits caused by prenatal exposure to ethanol. Nutrients 9, 1080 (2017).
Zeisel, S. H. Nutrition in pregnancy: the argument for including a source of choline. Int J. Women’s Health 5, 193–199 (2013).
Schneider, J. S. et al. Parkinson’s disease: improved function with GM1 ganglioside treatment in a randomized placebo-controlled study. Neurology 50, 1630–1636 (1998).
Kalpoyiannis, N. et al. Efficacy of phototherapy and/or exchange transfusions in neonatal jaundice. Clin. Pediatr. 21, 602–606 (1982).
Geiger, A. S., Rice, A. C. & Shapiro, S. M. Minocycline blocks acute bilirubin-induced neurological dysfunction in jaundiced Gunn rats. Neonatology 92, 219–226 (2007).
Acknowledgements
This material is original and has not been previously published nor has it been submitted for publication elsewhere while under consideration. We would like to acknowledge the special contributions of Sean Sukys, Hemal N. Sempat, and Nicholas Rickman. This study was funded by NIH R01AA016398 and R21HD085061, Rockville, MD (CB) and the Cobey Endowment, Baltimore, MD (CB) and The Munro Fund, Baltimore, MD (CB).
Author information
Authors and Affiliations
Contributions
S.T.K. substantially contributed to the analysis and interpretation of data, and drafting and revising of this article. N.T. and M.H. substantially contributed to the design of this study and the acquisition and analysis of data in this article. E.L. substantially contributed to the drafting of this article and the acquisition of data in this article. S.M.M. substantially contributed to the design of this study and the revising of this article. C.F.B. substantially contributed to the conception and design of this study and has final approval of the version to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kitchen, S.T., Tang, N., He, M. et al. Bilirubin inhibits lipid raft dependent functions of L1 cell adhesion molecule in rat pup cerebellar granule neurons. Pediatr Res 89, 1389–1395 (2021). https://doi.org/10.1038/s41390-020-01156-0
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-020-01156-0
This article is cited by
-
Choline attenuates bilirubin induced effects on tyrosine phosphorylation and distribution in lipid rafts of L1 cell adhesion molecule in vivo
Pediatric Research (2026)
-
Choline supplementation mitigates effects of bilirubin in cerebellar granule neurons in vitro
Pediatric Research (2024)
-
Neonatal hypoxia ischemia redistributes L1 cell adhesion molecule into rat cerebellar lipid rafts
Pediatric Research (2022)