Abstract
Background
Preterm birth places infants at higher risk of adverse long-term behavioral and cognitive outcomes. Combining biobehavioral measures and molecular biomarkers may improve tools to predict the risk of long-term developmental delays.
Methods
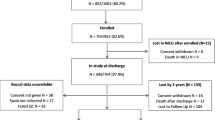
The Neonatal Neurobehavior and Outcomes in Very Preterm Infants study was conducted at nine neonatal intensive care units between April 2014 and June 2016. Cries were recorded and buccal swabs collected during the neurobehavioral exam. Cry episodes were extracted and analyzed using a computer system and the data were summarized using factor analysis. Genomic DNA was extracted from buccal swabs, quantified using the Qubit Fluorometer, and aliquoted into standardized concentrations. DNA methylation was measured with the Illumina MethylationEPIC BeadArray, and an epigenome-wide association study was performed using cry factors (n = 335).
Results
Eighteen CpGs were associated with the cry factors at genome-wide significance (α = 7.08E − 09). Two CpG sites, one intergenic and one linked to gene TCF3 (important for B and T lymphocyte development), were associated with acoustic measures of cry energy. Increased methylation of TCF3 was associated with a lower energy-related cry factor. We also found that pitch (F0) and hyperpitch (F0 > 1 kHz) were associated with DNA methylation variability at 16 CpG sites.
Conclusions
Acoustic cry characteristics are related to variation in DNA methylation in preterm infants.
Impact
-
Preterm birth is a major public health problem and its long-term impact on health is not well understood.
-
Cry acoustics, related to prematurity, has been linked to a variety of medical conditions.
-
Biobehavioral measures and molecular biomarkers can improve prediction tools for long-term developmental risks of preterm birth.
-
Variation in epigenetic modulation in preterm infants provides a potential link between preterm birth and unfavorable developmental outcomes.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Purisch, S. E. & Gyamfi-Bannerman, C. Epidemiology of preterm birth. Semin. Perinatol. 41, 387–391 (2017).
Institute of Medicine (US) Committee on Understanding Premature Birth and Assuring Healthy Outcomes. in Preterm Birth: Causes, Consequences, and Prevention, The National Academies Collection: Reports funded by National Institutes of Health (eds Behrman, R. E. & Butler, A. S.) (National Academies Press, Washington, 2007).
Aylward, G. P. Neurodevelopmental outcomes of infants born prematurely. J. Dev. Behav. Pediatr. 35, 394–407 (2014).
Amer, R. Neurodevelopmental outcomes of infants born at <29 weeks of gestation admitted to Canadian Neonatal Intensive Care Units based on location of birth. J. Pediatr. 196, 31–37 (2018).
Rogers, E. E. Early neurodevelopmental outcomes of extremely preterm infants. Semin. Perinatol. 40, 497–509 (2017).
LaGasse, L. L., Neal, A. R. & Lester, B. M. Assessment of infant cry: acoustic cry analysis and parental perception. Mental Retard. Dev. Disab. Res. Rev. 11, 83–93 (2005).
Sheinkopf, S. J., Iverson, J. M., Rinaldi, M. L. & Lester, B. M. Atypical cry acoustics in 6-month-old infants at risk for autism spectrum disorder. Autism Res. 5, 331–339 (2012).
Provenzi, L. et al. Pain-related stress during the Neonatal Intensive Care Unit stay and SLC6A4 methylation in very preterm infants. Front. Behav. Neurosci. 9, 99 (2015).
Lester, B. M. et al. Neurobehavior related to epigenetic differences in preterm infants. Epigenomics 7, 1123–1136 (2015).
Tendl, K. A. et al. DNA methylation pattern of CALCA in preterm neonates with bacterial sepsis as a putative epigenetic biomarker. Epigenetics 8, 1261–1267 (2013).
Sheinkopf, S. J., Righi, G., Marsit, C. J. & Lester, B. M. Methylation of the glucocorticoid receptor (NR3C1) in placenta is associated with infant cry acoustics. Front. Behav. Neurosci. 10, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4889592/ (2016).
Everson, T. M. et al. Epigenome-wide analysis identifies genes and pathways linked to neurobehavioral variation in preterm infants. Sci. Rep. 9, 6322 (2019).
Walden, R. V. et al. Major congenital anomalies place extremely low birth weight infants at higher risk for poor growth and developmental outcomes. Pediatrics 120, e1512–e1519 (2007).
Child Mind Institute. Hollingshead Four-Factor Index of Socioeconomic Status (SES-Child). Sample documentation (Nathan Kline Institute, Rockland, 2019).
Teschendorff, A. E. et al. A beta-mixture quantile normalization method for correcting probe design bias in Illumina Infinium 450 k DNA methylation data. Bioinformatics 29, 189–196 (2013).
Pidsley, R. et al. Critical evaluation of the Illumina MethylationEPIC BeadChip microarray for whole-genome DNA methylation profiling. Genome Biol. 17, 208 (2016).
Jaffe, A. E. & Irizarry, R. A. Accounting for cellular heterogeneity is critical in epigenome-wide association studies. Genome Biol. 15, R31 (2014).
Zheng, S. C. et al. A novel cell-type deconvolution algorithm reveals substantial contamination by immune cells in saliva, buccal and cervix. Epigenomics 10, 925–940 (2018).
Reggiannini, B., Sheinkopf, S. J., Silverman, H. F., Li, X. & Lester, B. M. A flexible analysis tool for the quantitative acoustic assessment of infant cry. J. Speech Lang. Hear. Res. 56, 1416–1428 (2013).
Ruscio, J. & Roche, B. Determining the number of factors to retain in an exploratory factor analysis using comparison data of known factorial structure. Psychol. Assess. 24, 282–292 (2012).
Buniello, A. et al. The NHGRI-EBI GWAS Catalog of published genome-wide association studies, targeted arrays and summary statistics 2019. Nucleic Acids Res. 47, D1005–D1012 (2019).
Stelzer, G. et al. The GeneCards Suite: From Gene Data Mining to Disease Genome Sequence Analysis. Curr. Protoc. Bioinformatics 54, 1.30.1–1.30.33 (2016).
Koshibu, K. et al. Protein phosphatase 1 regulates the histone code for long-term memory. J. Neurosci. 29, 13079–13089 (2009).
Verdugo-Sivianes, E. M. et al. Coordinated downregulation of Spinophilin and the catalytic subunits of PP1, PPP1CA/B/C, contributes to a worse prognosis in lung cancer. Oncotarget 8, 105196–105210 (2017).
Nguyen, J. T. et al. Mammalian EAK-7 activates alternative mTOR signaling to regulate cell proliferation and migration. Sci. Adv. 4, eaao5838 (2018).
Paulsen, M. T. et al. The p53-targeting human phosphatase hCdc14A interacts with the Cdk1/cyclin B complex and is differentially expressed in human cancers. Mol. Cancer 5, 25 (2006).
Imtiaz, A. et al. CDC14A phosphatase is essential for hearing and male fertility in mouse and human. Hum. Mol. Genet. 27, 780–798 (2018).
Shen, G. et al. Overexpression of uridine-cytidine kinase 2 correlates with breast cancer progression and poor prognosis. J. Breast Cancer 20, 132–141 (2017).
Yu, S. et al. UCK2 upregulation might serve as an indicator of unfavorable prognosis of hepatocellular carcinoma. IUBMB Life 71, 105–112 (2019).
Somasundaram, R., Prasad, M., Ungerback, J. & Mikaek, S. Transcription factor networks in B-cell differentiation link development to acute lymphoid leukemia. Blood 126, 144–152 (2015).
Li, C., Cai, S., Wang, X. & Jiang, Z. Hypomethylation-associated up-regulation of TCF3 expression and recurrence in stage II and III colorectal cancer. PLoS ONE 9, e112005 (2014).
Basile, M. et al. Paralemmin interacts with D3 dopamine receptors: Implications for membrane localization and cAMP signaling. Arch. Biochem. Biophys. 446, 60–68 (2006).
Lester, B. M. & Boukydis, C. F. Z. No language but a cry. in Nonvebal Vocal Communication: Comparative and Developmental Approaches (eds Papoušek, H., Jurgens, U. & Papoušek, M.) 41–69 (Cambridge Univ. Press, 1992).
Lester, B. M. Developmental outcome prediction from acoustic cry analysis in term and preterm infants. Pediatrics 80, 529–534 (1987).
Lester, B. M. A biosocial model of infant crying. in Advances in Infant Research (eds Lipsitt, L. & Rovee-Collier, C.) 167–212 (Ablex, Norwood NJ, 1984).
Acknowledgements
This work was funded by National Institutes of Health (NIH)/Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Grant R01HD072267 (Lester and O’Shea), R01HD084515 (Lester and Everson), and UH3OD023347 (B.M.L., C.M., and T.M.O’S.), National Center for Advancing Translational Sciences through UCLA CTSI Grant UL1TR001881 and by the National Institute of Environmental Health Sciences HERCULES Center (P30 ES019776).
Author information
Authors and Affiliations
Contributions
G.A. designed the study, extracted and processed cry recordings, analyzed and interpreted data, drafted the article and revised critically for important intellectual content, and approved the final version as submitted. S.J.S. developed the acoustic cry system, reviewed and revised critically for important intellectual content, and approved the final version as submitted. C.J.M. performed the laboratory studies, interpreted methylation data, reviewed and revised critically for important intellectual content, and approved the final version as submitted. T.M.E. performed the laboratory studies, analyzed and interpreted methylation data, reviewed and revised critically for important intellectual content, and approved the final version as submitted. H.L. extracted and processed cry recordings, reviewed and revised critically for important intellectual content, and approved the final version as submitted. A.A.B. analyzed methylation data, reviewed and revised critically for important intellectual content, and approved the final version as submitted. B.S.C. reviewed and revised critically for important intellectual content and approved the final version as submitted. J.B.H. reviewed and revised critically for important intellectual content and approved the final version as submitted. J.A.H. reviewed and revised critically for important intellectual content and approved the final version as submitted. E.C.McG. reviewed and revised critically for important intellectual content and approved the final version as submitted. C.R.N. reviewed and revised critically for important intellectual content and approved the final version as submitted. T.M.O’S. conceptualized and designed the study, reviewed and revised critically for important intellectual content, and approved the final version as submitted. S.L.P. reviewed and revised critically for important intellectual content and approved the final version as submitted. L.M.S. reviewed and revised critically for important intellectual content and approved the final version as submitted. A.S. reviewed and revised critically for important intellectual content and approved the final version as submitted. L.M.D. analyzed and interpreted data, reviewed and revised critically for important intellectual content, and approved the final version as submitted. S.A.D. coordinated data collection, reviewed and revised critically for important intellectual content, and approved the final version as submitted. Dr. J.F.P. coordinated laboratory analyses, reviewed and revised critically for important intellectual content, and approved the final version as submitted. B.M.L. conceptualized and designed the study, developed the acoustic cry system, interpreted data, drafted the article and revised critically for important intellectual content, and approved the final version as submitted.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Statement of consent
The study was approved by Western Institutional Review Board, Puyallup, WA (HI site), the John F. Wolf Human Subjects Committee, Los Angeles, CA, Spectrum Health Systems, Inc. IRB, Grand Rapids, MI, Wake Forest University Health Sciences IRB, Winston-Salem, NC, Children’s Mercy Hospital IRB, Kansas City, MO, Women & Infants Hospital IRB, Providence, RI.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Aghagoli, G., Sheinkopf, S.J., Everson, T.M. et al. Epigenome-wide analysis identifies genes and pathways linked to acoustic cry variation in preterm infants. Pediatr Res 89, 1848–1854 (2021). https://doi.org/10.1038/s41390-020-01172-0
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-020-01172-0
This article is cited by
-
Associations of maternal night shift work during pregnancy with DNA methylation in offspring: a meta-analysis in the PACE consortium
Clinical Epigenetics (2025)
-
Epigenome-wide association study identifies neonatal DNA methylation associated with two-year attention problems in children born very preterm
Translational Psychiatry (2024)
-
Environmental influences on child health outcomes: cohorts of individuals born very preterm
Pediatric Research (2023)