Abstract
Background
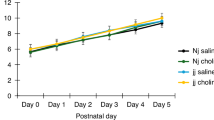
Bilirubin is produced by the breakdown of hemoglobin and is normally catabolized and excreted. Neurotoxic accumulation of serum bilirubin often occurs in premature infants. The homozygous Gunn rat lacks uridine diphosphate glucuronosyltransferase 1A1 (UGT1A1), the enzyme needed to biotransform bilirubin. This rodent model of hyperbilirubinemia emulates many aspects of bilirubin toxicity observed in the human infant. We demonstrate that choline supplementation in early postnatal development is neuroprotective in the choline-restricted Gunn rat, when hyperbilirubinemia is induced on postnatal day 5.
Methods
We first compared behaviors and cerebellar weight of pups born to dams consuming regular rat chow to those of dams consuming choline-restricted diets. Second, we measured behaviors and cerebellar weights of pups born to choline-restricted dams, reared on a choline-restricted diet, supplemented with or without choline, and treated with or without sulfadimethoxine (SDMX).
Results
A choline-restricted diet did not change the behavioral outcomes, but cerebellar weight was reduced in the choline-restricted group regardless of genotype or SDMX administration. SDMX induced behavioral deficits in jj pups, and choline supplementation improved most behavioral effects and cerebellar weight in SDMX-treated jj rats.
Conclusions
These results suggest that choline may be used as a safe and effective neuroprotective intervention against hyperbilirubinemia in the choline-deficient premature infant.
Impact
-
This article investigates the effect of neonatal jaundice/bilirubin neurotoxicity on cerebellar-mediated behaviors.
-
This article explores the potential use of choline as an intervention capable of ameliorating the effect of bilirubin on the choline-restricted developing brain.
-
This article opens the door for future studies on the action of choline in the presence of hyperbilirubinemia, especially in preterm neonates.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
van der Schoor, L. W. et al. Unconjugated free bilirubin in preterm infants. Early Hum. Dev. 106–107, 25–32 (2017).
Bhutani, V. K., Wong, R. J. & Stevenson, D. K. Hyperbilirubinemia in preterm neonates. Clin. Perinatol. 43, 215–232 (2016).
Amin, S. B. & Wang, H. Bilirubin albumin binding and unbound unconjugated hyperbilirubinemia in premature infants. J. Pediatr. 192, 47–52 (2018).
Watchko, J. F. & Jeffrey Maisels, M. The enigma of low bilirubin kernicterus in premature infants: why does it still occur, and is it preventable? Semin. Perinatol. 38, 397–406 (2014).
Lin, S. et al. Minocycline blocks bilirubin neurotoxicity and prevents hyperbilirubinemia-induced cerebellar hypoplasia in the Gunn rat. Eur. J. Neurosci. 22, 21–27 (2005).
Robert, M. C. et al. Alterations in the cell cycle in the cerebellum of hyperbilirubinemic Gunn rat: a possible link with apoptosis? PLoS ONE 8, e79073 (2013).
Rodrigues, C. M. P. et al. Perturbation of membrane dynamics in nerve cells as an early event during bilirubin-induced apoptosis. J. Lipid Res 43, 885–894 (2002).
Rodrigues, C. M. P., Solá, S., Brito, M. A., Brites, D. & Moura, J. J. G. Bilirubin directly disrupts membrane lipid polarity and fluidity, protein order, and redox status in rat mitochondria. J. Hepatol. 36, 335–341 (2002).
Watchko, J. F. Kernicterus and the molecular mechanisms of bilirubin-induced CNS injury in newborns. Neuromolecular Med. 8, 513–529 (2006).
Altman, J. Morphological development of rat cerebellum and some of its mechanisms. Exp. Brain Res. (Suppl. 6) 8–49 (1982).
Chang, C. H., Chang, F. M., Yu, C. H., Ko, H. C. & Chen, H. Y. Assessment of fetal cerebellar volume using three-dimensional ultrasound. Ultrasound Med. Biol. 26, 981–988 (2000).
Volpe, J. J. Cerebellum of the premature infant: rapidly developing, vulnerable, clinically important. J. Child Neurol. 24, 1085–1104 (2009).
Rakic, P. & Sidman, R. L. Histogenesis of cortical layers in human cerebellum, particularly the lamina dissecans. J. Comp. Neurol. 139, 473–500 (1970).
Sidman, R. L. & Rakic, P. Neuronal migration, with special reference to developing human brain: a review. Brain Res. 62, 1–35 (1973).
Hashimoto, K., Ichikawa, R., Kitamura, K., Watanabe, M. & Kano, M. Translocation of a “winner” climbing fiber to the Purkinje cell dendrite and subsequent elimination of “losers” from the soma in developing cerebellum. Neuron 63, 106–118 (2009).
Watanabe, M. & Kano, M. Climbing fiber synapse elimination in cerebellar Purkinje cells. Eur. J. Neurosci. 34, 1697–1710 (2011).
Cannon, C., Daood, M. J., O’Day, T. L. & Watchko, J. F. Sex-specific regional brain bilirubin content in hyperbilirubinemic Gunn rat pups. Biol. Neonate 90, 40–45 (2006).
Conlee, J. W. & Shapiro, S. M. Development of cerebellar hypoplasia in jaundiced Gunn rats: a quantitative light microscopic analysis. Acta Neuropathol. 93, 450–460 (1997).
Waddell, J., He, M., Tang, N., Rizzuto, C. & Bearer, C. F. A Gunn rat model of preterm hyperbilirubinemia. Pediatr. Res. 87, 480–484 (2020).
Johnson, L., Sarmiento, F., Blanc, W. A. & Day, R. Kernicterus in rats with an inherited deficiency of glucuronyl transferase. Am. J. Dis. Child. 97, 591–608 (1959).
Yamamura, H. & Takagishi, Y. Cerebellar hypoplasia in the hyperbilirubinemic Gunn rat: morphological aspects. Nagoya J. Med. Sci. 55, 11–21 (1993).
Schutta, H. S. & Johnson, L. Clinical signs and morphologic abnormalities in Gunn rats treated with sulfadimethoxine. J. Pediatr. 75, 1070–1079 (1969).
Davis, N. L., Tang, N., He, M., Lee, D. & Bearer, C. F. Choline ameliorates ethanol induced alterations in tyrosine phosphorylation and distribution in detergent-resistant membrane microdomains of L1 cell adhesion molecule in vivo. Birth Defects Res. 112, 480–489 (2020).
Tang, N. et al. Ethanol causes the redistribution of L1 cell adhesion molecule in lipid rafts. J. Neurochem. 119, 859–867 (2011).
Watchko, J. F. Bilirubin-induced neurotoxicity in the preterm neonate. Clin. Perinatol. 43, 297–311 (2016).
Bearer, C. F. F., Wellmann, K. A. A., Tang, N., He, M. & Mooney, S. M. M. Choline ameliorates deficits in balance caused by acute neonatal ethanol exposure. Cerebellum 14, 413–420 (2015).
Gallego-Ortega, D. et al. Differential role of human choline kinase alpha and beta enzymes in lipid metabolism: implications in cancer onset and treatment. PLoS ONE 4, e7819 (2009).
Wu, G. & Vance, D. E. Choline kinase and its function. Biochem. Cell Biol. 88, 559–564 (2010).
Zeisel, S. H. & da Costa, K. A. Choline: an essential nutrient for public health. Nutr. Rev. 67, 615–623 (2009).
Niculescu, M. D. & Zeisel, S. H. Diet, methyl donors and DNA methylation: interactions between dietary folate, methionine and choline. J. Nutr. 132, 2333S–2335S (2002).
Mehedint, M. G., Niculescu, M. D., Craciunescu, C. N. & Zeisel, S. H. Choline deficiency alters global histone methylation and epigenetic marking at the Re1 site of the calbindin 1 gene. FASEB J. 24, 184–195 (2010).
Zeisel, S. H. Nutritional genomics: defining the dietary requirement and effects of choline. J. Nutr. 141, 531–534 (2011).
Branchi, I., Santucci, D. & Alleva, E. Ultrasonic vocalisation emitted by infant rodents: a tool for assessment of neurobehavioural development. Behav. Brain Res. 125, 49–56 (2001).
Krüger, H. S., Brockmann, M. D., Salamon, J., Ittrich, H. & Hanganu-Opatz, I. L. Neonatal hippocampal lesion alters the functional maturation of the prefrontal cortex and the early cognitive development in pre-juvenile rats. Neurobiol. Learn. Mem. 97, 470–481 (2012).
Silkstone, M. & Brudzynski, S. M. Dissimilar interaction between dopaminergic and cholinergic systems in the initiation of emission of 50-kHz and 22-kHz vocalizations. Pharmacol. Biochem Behav. 188, 172815 (2020).
Nascimento, A. F. et al. Lipopolysaccharide-induced sickness behavior in lactating rats decreases Ultrasonic Vocalizations and exacerbates immune system activity in male offspring. Neuroimmunomodulation 22, 213–221 (2015).
Ulus, I. H., Wurtman, R. J., Mauron, C. & Blusztajn, J. K. Choline increases acetylcholine release and protects against the stimulation-induced decrease in phosphatide levels within membranes of rat corpus striatum. Brain Res. 484, 217–227 (1989).
Meck, W. H. & Williams, C. L. Characterization of the facilitative effects of perinatal choline supplementation on timing and temporal memory. Neuroreport 8, 2831–2835 (1997).
Goodlett, C. R., Thomas, J. D. & West, J. R. Long-term deficits in cerebellar growth and rotarod performance of rats following “binge-like” alcohol exposure during the neonatal brain growth spurt. Neurotoxicol. Teratol. 13, 69–74 (1991).
Hamm, R. J., Pike, B. R., O’dell, D. M., Lyeth, B. G. & Jenkins, L. W. The rotarod test: an evaluation of its effectiveness in assessing motor deficits following traumatic brain injury. J. Neurotrauma 11, 187–196 (1994).
Sakayori, N. et al. Motor skills mediated through cerebellothalamic tracts projecting to the central lateral nucleus. Mol. Brain 12, 13 (2019).
Soreq, H. Checks and balances on cholinergic signaling in brain and body function. Trends Neurosci. 38, 448–458 (2015).
Kljakic, O., Janickova, H., Prado, V. F. & Prado, M. A. M. Cholinergic/glutamatergic co-transmission in striatal cholinergic interneurons: new mechanisms regulating striatal computation. J. Neurochem. 142, 90–102 (2017).
Nakai, Y. & Kamiguchi, H. Migration of nerve growth cones requires detergent-resistant membranes in a spatially defined and substrate-dependent manner. J. Cell Biol. 159, 1097–1108 (2002).
Tang, N. et al. Choline partially prevents the impact of ethanol on the lipid raft dependent functions of l1 cell adhesion molecule. Alcohol Clin. Exp. Res. 38, 2722–2730 (2014).
Bernhard, W. et al. Choline supply of preterm infants: assessment of dietary intake and pathophysiological considerations. Eur. J. Nutr. 52, 1269–1278 (2013).
Bernhard, W. et al. Combined choline and DHA supplementation: a randomized controlled trial. Eur. J. Nutr. 59, 729–739 (2020).
Das, S. & van Landeghem, F. K. H. Clinicopathological spectrum of bilirubin encephalopathy/kernicterus. Diagnostics 9, 24 (2019).
Meck. W. H. & Williams, C. L. Metabolic imprinting of choline by its availability during gestation: implications for memory and attentional processing across the lifespan. Neurosci. Biobehav. Rev. 27, 385–399 (2003).
Acknowledgements
This work was supported by the NIH/NICHD R21HD085061.
Author information
Authors and Affiliations
Contributions
J.W.: substantial contributions to analysis and interpretation of data, drafting and revising article critically for important intellectual content, and final approval of the version to be published; N.C.R.: drafting the article or revising it critically for important intellectual content; M.H.: substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; N.T.: substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; C.F.B.: substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; drafting the article or revising it critically for important intellectual content; and final approval of the version to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Waddell, J., Rickman, N.C., He, M. et al. Choline supplementation prevents the effects of bilirubin on cerebellar-mediated behavior in choline-restricted Gunn rat pups. Pediatr Res 89, 1414–1419 (2021). https://doi.org/10.1038/s41390-020-01187-7
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-020-01187-7
This article is cited by
-
Choline attenuates bilirubin induced effects on tyrosine phosphorylation and distribution in lipid rafts of L1 cell adhesion molecule in vivo
Pediatric Research (2026)
-
Choline supplementation mitigates effects of bilirubin in cerebellar granule neurons in vitro
Pediatric Research (2024)
-
Models of bilirubin neurological damage: lessons learned and new challenges
Pediatric Research (2023)