Abstract
Background
Transport of iron across the placenta is critical for appropriate development of the fetus. Iron deficiency during pregnancy remains a major public health concern, particularly in low- and middle-income countries, often exacerbated by infectious diseases leading to altered iron trafficking via inflammatory responses. Herein, we investigate the role of hepcidin, a master regulator of iron homeostasis, on regulation of iron transport across trophoblast cells.
Methods
We utilized the Jeg-3 choriocarcinoma cell line for analysis of the expression of transferrin receptor, ferritin, and ferroportin as well as the export of 59Fe in the presence of hepcidin. Placental tissue from human term pregnancies was utilized for immunohistochemistry.
Results
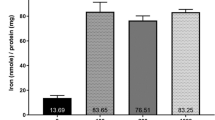
Hepcidin treatment of Jeg-3 cells decreased the expression of ferroportin and transferrin receptor (TfR) and reduced the cellular export of iron. Lower expression of TfR on the syncytiotrophoblast was associated with the highest levels of hepcidin in maternal circulation, and ferroportin expression was positively associated with placental TfR. Placentas from small-for-gestational-age newborns had significantly lower levels of ferroportin and ferritin gene expression at delivery.
Conclusions
Our data suggest that hepcidin plays an important role in the regulation of iron transport across the placenta, making it a critical link in movement of iron into fetal circulation.
Impact
-
Hepcidin has a direct impact on iron transport across the human placenta.
-
This study provides the first evidence of direct regulation of iron efflux from human trophoblast cells by hepcidin.
-
These data extend our understanding of iron transport across the maternal–fetal interface, a process critical for fetal health and development.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Fomon, S. J., Nelson, S. E. & Ziegler, E. E. Retention of iron by infants. Annu. Rev. Nutr. 20, 273–290 (2000).
Kretchmer, N., Beard, J. L. & Carlson, S. The role of nutrition in the development of normal cognition. Am. J. Clin. Nutr. 63, 997S–1001S (1996).
McLean, E. et al. Worldwide prevalence of anaemia, WHO Vitamin and Mineral Nutrition Information System, 1993-2005. Public Health Nutr. 12, 444–454 (2008).
Lozoff, B. & Georgieff, M. K. Iron deficiency and brain development. Semin. Pediatr. Neurol. 13, 158–165 (2006).
Clardy, S. L. et al. Acute and chronic effects of developmental iron deficiency on mRNA expression patterns in the brain. J. Neural. Transm. Suppl. 173–196 (2006).
Felt, B. T. & Lozoff, B. Brain iron and behavior of rats are not normalized by treatment of iron deficiency anemia during early development. J. Nutr. 126, 693–701 (1996).
de Deungria, M. et al. Perinatal iron deficiency decreases cytochrome c oxidase (CytOx) activity in selected regions of neonatal rat brain. Pediatr. Res. 48, 169–176 (2000).
Beard, J. L. Iron biology in immune function, muscle metabolism and neuronal functioning. J. Nutr. 131, 568S–579S (2001). Discussion 580S.
Rao, R. & Georgieff, M. K. Neonatal iron nutrition. Semin. Neonatol. 6, 425–435 (2001).
Ganz, T. Hepcidin, a key regulator of iron metabolism and mediator of anemia of inflammation. Blood 102, 783–788 (2003).
Nicolas, G. et al. Lack of hepcidin gene expression and sever tissue iron overload in upstream stimulatory factor 2 (USF2) knockout mice. Proc. Natl Acad. Sci. USA 98, 8780–8785 (2001).
Nicolas, G. et al. Severe iron deficiency anemia in transgenic mice expressing liver hepcidin. Proc. Natl Acad. Sci. USA 99, 4596–4601 (2002).
Park, C., Valore, E., Waring, A. & Ganz, T. Hepcidin, a urinary antimicrobial peptide synthesized in the liver. J. Biol. Chem. 276, 7806–7810 (2001).
Krause, A. et al. LEAP-1, a novel highly disulfide-bonded human peptide, exhibits antimicrobial activity. FEBS Lett. 480, 147–150 (2000).
Pigeon, C. et al. A new mouse liver-specific gene, encoding a protein homologous to human antimicrobial peptide hepcidin, is overexpressed during iron overload. J. Biol. Chem. 276, 7811–7819 (2001).
Nemeth, E. et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 306, 2090–2093 (2004).
Delaby, C. et al. Presence of the iron exporter ferroportin at the plasma membrane of macrophages is enhanced by iron loading and down-regulated by hepcidin. Blood 106, 3979–3984 (2005).
Abboud, S. & Haile, D. J. A novel mammalian iron-regulated protein involved in intracellular iron metabolism. J. Biol. Chem. 275, 19906–19912 (2000).
McKie, A. T. et al. A novel duodenal iron-regulated transporter, IREG1, implicated in the basolateral transfer of iron to the circulation. Mol. Cell 5, 299–309 (2000).
Donovan, A. et al. Positional cloning of zebrafish ferroportin1 identifies a conserved vertebrate iron exporter. Nature 403, 776–781 (2000).
Bradley, J. et al. Influence of gestational age and fetal iron status on IRP activity and iron transporter protein expression in third-trimester human placenta. Am. J. Physiol. Regul. Integr. Comp. Physiol. 287, R894–R901 (2004).
Sangkhae, V. et al. Effects of maternal iron status on placental and fetal iron homeostasis. J. Clin. Investig. 130, 625–640 (2020).
Georgieff, M. K., Wewerka, S. W., Nelson, C. A. & Deregnier, R. A. Iron status at 9 months of infants with low iron stores at birth. J. Pediatr. 141, 405–409 (2002).
Olveda, R. et al. Efficacy and safety of praziquantel for the treatment of human schistosomiasis during pregnancy: a phase 2, randomised, double-blind, placebo-controlled trial. Lancet Infect. Dis. 16, 199–208 (2016).
Villar, J. et al. International standards for newborn weight, length, and head circumference by gestational age and sex: the Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. Lancet 384, 857–868 (2014).
World Health Organization. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. Vitamin and Mineral Nutrition Information System: WHO/NMH/NHD/MNM/11.11. https://www.who.int/vmnis/indicators/haemoglobin/en/ (2011).
Coutinho, H. et al. Nutritional status and serum cytokine profiles in children, adolescents, and young adults with Schistosoma japonicum-associated hepatic fibrosis, in Leyte, Philippines. J. Infect. Dis. 192, 528–536 (2005).
Cao, C. & Fleming, M. The placenta: the forgotten essential organ of iron transport. Nutr. Rev. 74, 421–431 (2016).
Douglas, G. & King, B. Uptake and processing of 125I-labelled transferring and 59Fe-labelled transferrin by isolated human trophoblast cells. Placenta 11, 41–57 (1990).
Bastin, J. et al. Localisation of proteins of iron metabolism in the human placenta and liver. Br. J. Haematol. 134, 532–543 (2006).
Mok, H. et al. Dysregulation of ferroportin 1 interferes with spleen organogenesis in polychthaemia mice. Development 131, 4871–4881 (2004).
Donovan, A. et al. The iron exporter ferroportin/Slc40a1 is essential for iron homeostasis. Cell Metab. 1, 191–200 (2005).
Mao, J. et al. The iron exporter ferroportin 1 is essential for development of the mouse embryo, forebrain patterning and neural tube closure. Development 137, 3079–3088 (2010).
Young, M. F. et al. Maternal hepcidin is associated with placental transfer of iron derived from dietary heme and nonheme sources. J. Nutr. 142, 33–39 (2012).
Bah, A. et al. Serum hepcidin concentrations decline during pregnancy and may identify iron deficiency: analysis of a longitudinal pregnancy cohort in the Gambia. J. Nutr. 147, 1131–1137 (2017).
Evans, P. et al. Hepcidin and iron species distribution inside the first-trimester human gestational sac. Mol. Hum. Reprod. 17, 227–232 (2011).
Martin, M. et al. Transferrin receptor 1 mRNA is downregulated in placenta of hepcidin transgenic embryos. FEBS Lett. 574, 187–191 (2004).
Gambling, L. et al. Fetal iron status regulates maternal iron metabolism during pregnancy in the rat. Am. J. Physiol. Regul. Integr. Comp. Physiol. 296, R1063–R1070 (2009).
Garcia-Valdes, L. et al. The impact of maternal obesity on iron status, placental transferrin receptor expression and hepcidin expression in human pregnancy. Int. J. Obes. 39, 571–578 (2015).
Young, M. et al. Impact of maternal and neonatal iron status on placental transferrin receptor expression in pregnant adolescents. Placenta 31, 1010–1014 (2010).
Abioye, A. et al. Anemia of inflammation during human pregnancy does not affect newborn iron endowment. J. Nutr. 148, 427–436 (2018).
Lubach, G. & Coe, C. Preconception maternal iron status is a risk factor for iron deficiency in infant rhesus monkeys (Macaca mulatta). J. Nutr. 136, 2345–2349 (2006).
Georgieff, M. K., Berry, S. A., Wobken, J. D. & Leibold, E. A. Increased placental iron regulatory protein-1 expression in diabetic pregnancies complicated by fetal iron deficiency. Placenta 20, 87–93 (1999).
Balesaria, S. et al. Fetal iron levels are regulated by maternal and fetal Hfe genotype and dietary iron. Haemtologica 97, 661–669 (2012).
Willemetz, A. et al. Matriptase-2 is essential for hepcidin repression during fetal life and postnatal development in mice to maintain iron homeostasis. Blood 124, 441–444 (2014).
Lee, S. et al. Prevalence of anemia and associations between neonatal iron status, hepcidin, and maternal iron status among neonates born to pregnant adolescents. Pediatr. Res. 79, 42–48 (2016).
Ervasti, M. et al. Maternal pro-hepcidin at term correlates with cord blood pro-hepcidin at birth. Eur. J. Obstet. Gynecol. Reprod. Biol. 147, 161–165 (2009).
Young, M. et al. Maternal hepcidin is associated with placental transfer of iron derived from dietary heme and nonheme sources. J. Nutr. 142, 33–39 (2012).
Koenig, M. D. et al. Hepcidin and iron homeostasis during pregnancy. Nutrients 6, 3062–3083 (2014).
Allen, L. Biological mechanisms that might underlie iron’s effects on fetal growth and preterm birth. J. Nutr. 131, 581S–589S (2001).
Mando, C. et al. Transferrin receptor gene and protein expression and localization in human IUGR and normal term placentas. Placenta 32, 44–50 (2011).
Gambling, L., Lang, C. & McArdle, H. J. Fetal regulation of iron transport during pregnancy. Am. J. Clin. Nutr. 94, 1903S–1907S (2011).
Rouault, T. A. The role of iron regulatory proteins in mammalian iron homeostasis and disease. Nat. Chem. Biol. 2, 406–414 (2006).
Acknowledgements
This work was supported by the National Institute of Allergy and Infectious Diseases [R21AI107520 to J.F.F. and K01AI13068 to E.A.M.] and by the National Institute of Diabetes and Digestive and Kidney Diseases [R01DK110049 to T.B.B.] at the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
Conception and design; acquisition; analysis or interpretation of data; and final approval: all authors; drafting of the article: E.A.M., T.B.B., J.D.K., J.F.F.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent
Written informed consent was obtained by women early in gestation, which included agreement to inclusion of their tissue in data analysis and publication.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
McDonald, E.A., Gundogan, F., Olveda, R.M. et al. Iron transport across the human placenta is regulated by hepcidin. Pediatr Res 92, 396–402 (2022). https://doi.org/10.1038/s41390-020-01201-y
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-020-01201-y
This article is cited by
-
Molecular insights into placental iron transfer mechanisms and maternofetal regulation
Archives of Gynecology and Obstetrics (2023)
-
Fetoplacental oxygen homeostasis in pregnancies with maternal diabetes mellitus and obesity
Nature Reviews Endocrinology (2022)
-
The effect of iron supplementation on maternal iron deficiency anemia does not differ by baseline anemia type among Tanzanian pregnant women without severe iron deficiency anemia
European Journal of Nutrition (2022)