Abstract
Background
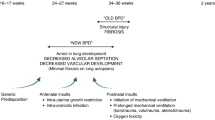
Chorioamnionitis, an intrauterine infection of the placenta and fetal membranes, is a common risk factor for adverse pulmonary outcomes in premature infants including BPD, which is characterized by an arrest in alveolar development. As endogenous epithelial stem/progenitor cells are crucial for organogenesis and tissue repair, we examined whether intrauterine inflammation negatively affects these essential progenitor pools.
Methods
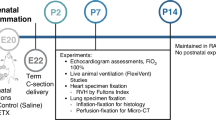
In an ovine chorioamnionitis model, fetuses were intra-amniotically exposed to LPS, 2d or 7d (acute inflammation) before preterm delivery at 125d of gestation, or to intra-amniotic Ureaplasma parvum for 42d (chronic inflammation). Lung function, pulmonary endogenous epithelial stem/progenitor pools, and downstream functional markers were studied.
Results
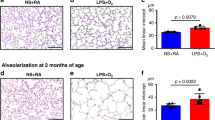
Lung function was improved in the 7d LPS and 42d Ureaplasma groups. However, intrauterine inflammation caused a loss of P63+ basal cells in proximal airways and reduced SOX-9 expression and TTF-1+ Club cells in distal airways. Attenuated type-2 cell numbers were associated with lower proliferation and reduced type-1 cell marker Aqp5 expression, indicative for impaired progenitor function. Chronic Ureaplasma infection only affected distal airways, whereas acute inflammation affected stem/progenitor populations throughout the lungs.
Conclusions
Acute and chronic prenatal inflammation improve lung function at the expense of stem/progenitor alterations that potentially disrupt normal lung development, thereby predisposing to adverse postnatal outcomes.
Impact
-
In this study, prenatal inflammation improved lung function at the expense of stem/progenitor alterations that potentially disrupt normal lung development, thereby predisposing to adverse postnatal outcomes.
-
Importantly, we demonstrate that these essential alterations can already be initiated before birth. So far, stem/progenitor dysfunction has only been shown postnatally.
-
This study indicates that clinical protocols to target the consequences of perinatal inflammatory stress for the immature lungs should be initiated as early as possible and ideally in utero. Within this context, our data suggest that interventions, which promote function or repair of endogenous stem cells in the lungs, hold great promise.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Sabic, D. & Koenig, J. M. A perfect storm: fetal inflammation and the developing immune system. Pediatr. Res. 87, 319–326 (2019).
Peng, C. C. et al. Intrauterine inflammation, infection, or both (Triple I): a new concept for chorioamnionitis. Pediatr. Neonatol. 59, 231–237 (2018).
Berger, A. et al. Microbial invasion of the amniotic cavity at birth is associated with adverse short-term outcome of preterm infants. J. Perinat. Med. 31, 115–121 (2003).
Galinsky, R. et al. The consequences of chorioamnionitis: preterm birth and effects on development. J. Pregnancy 2013, 412831 (2013).
Thomas, W. & Speer, C. P. Chorioamnionitis is essential in the evolution of bronchopulmonary dysplasia–the case in favour. Paediatr. Respir. Rev. 15, 49–52 (2014).
Alvira, C. M. & Morty, R. E. Can we understand the pathobiology of bronchopulmonary dysplasia? J. Pediatr. 190, 27–37 (2017).
Möbius, M. A. & Thébaud, B. Bronchopulmonary dysplasia: where have all the stem cells gone?: origin and (potential) function of resident lung stem cells. Chest 152, 1043–1052 (2017).
Collins, J. J. & Thebaud, B. Progenitor cells of the distal lung and their potential role in neonatal lung disease. Birth Defects Res. A Clin. Mol. Teratol. 100, 217–226 (2014).
Stahlman, M. T., Gray, M. E. & Whitsett, J. A. Expression of thyroid transcription factor-1 (TTF-1) in fetal and neonatal human lung. J. Histochem. Cytochem. 44, 673–678 (1996).
Yee, M. et al. Alternative progenitor lineages regenerate the adult lung depleted of alveolar epithelial type 2 cells. Am. J. Respir. Cell Mol. Biol. 56, 453–464 (2017).
Das, I. et al. A study of spectrum of pulmonary pathology and expression of thyroid transcription factor-1 during neonatal period. Indian J. Pathol. Microbiol. 61, 334–338 (2018).
Willems, M. G. et al. Pulmonary vascular changes in extremely preterm sheep after intra-amniotic exposure to Ureaplasma parvum and lipopolysaccharide. PLoS ONE 12, e0180114 (2017).
Kuypers, E. et al. Altered canonical Wingless-Int signaling in the ovine fetal lung after exposure to intra-amniotic lipopolysaccharide and antenatal betamethasone. Pediatr. Res. 75, 281–287 (2014).
Gussenhoven, R. et al. The paradoxical effects of chronic intra-amniotic Ureaplasma parvum exposure on ovine fetal brain development. Dev. Neurosci. 39, 472–486 (2017).
Willems, M. G. et al. Systemic interleukin-2 administration improves lung function and modulates chorioamnionitis-induced pulmonary inflammation in the ovine fetus. Am. J. Physiol. Lung Cell. Mol. Physiol. 310, L1–L7 (2016).
Leibel, S. & Post, M. Endogenous and exogenous stem/progenitor cells in the lung and their role in the pathogenesis and treatment of pediatric lung disease. Front. Pediatr. 4, 36 (2016).
Peake, J. L. & Pinkerton, K. E. in Comparative Biology of the Normal Lung (ed. Parent, R. A.) Ch. 3 (Elsevier, 2015).
Vaughan, A. E. et al. Lineage-negative progenitors mobilize to regenerate lung epithelium after major injury. Nature 517, 621–625 (2015).
Danopoulos, S. et al. Human lung branching morphogenesis is orchestrated by the spatiotemporal distribution of ACTA2, SOX2, and SOX9. Am. J. Physiol. Lung Cell. Mol. Physiol. 314, L144–L149 (2018).
Bianco-Miotto, T. et al. Epigenetics and DOHaD: from basics to birth and beyond. J. Dev. Orig. Health Dis. 8, 513–519 (2017).
Postma, D. S., Bush, A. & van den Berge, M. Risk factors and early origins of chronic obstructive pulmonary disease. Lancet 385, 899–909 (2015).
Um-Bergstrom, P. et al. Pulmonary outcomes in adults with a history of bronchopulmonary dysplasia differ from patients with asthma. Respir. Res. 20, 102 (2019).
Borghesi, A. et al. Circulating endothelial progenitor cells in preterm infants with bronchopulmonary dysplasia. Am. J. Respir. Crit. Care Med. 180, 540–546 (2009).
Schittny, J. C. Development of the lung. Cell Tissue Res. 367, 427–444 (2017).
Rennard, S. I. et al. Airway epithelial cells: functional roles in airway disease. Am. J. Respir. Crit. Care Med. 150, S27–S30 (1994).
Davies, D. E. Epithelial barrier function and immunity in asthma. Ann. Am. Thorac. Soc. 11, S244–S251 (2014).
Donne, M. L., Lechner, A. J. & Rock, J. R. Evidence for lung epithelial stem cell niches. BMC Dev. Biol. 15, 32 (2015).
Ota, C. et al. Linking bronchopulmonary dysplasia to adult chronic lung diseases: role of WNT signaling. Mol. Cell Pediatr. 3, 34 (2016).
Saito, A., Horie, M. & Nagase, T. TGF-beta signaling in lung health and disease. Int. J. Mol. Sci. 19, 2460 (2018).
Ahmed, A. S. et al. Effect of aging on stem cells. World J. Exp. Med. 7, 1–10 (2017).
Meiners, S. & Hilgendorff, A. Early injury of the neonatal lung contributes to premature lung aging: a hypothesis. Mol. Cell. Pediatr. 3, 24 (2016).
Jobe, A. H. & Bancalari, E. Bronchopulmonary dysplasia. Am. J. Respir. Crit. Care Med. 163, 1723–1729 (2001).
Viscardi, R. et al. Disordered pulmonary myofibroblast distribution and elastin expression in preterm infants with Ureaplasma urealyticum pneumonitis. Pediatr. Dev. Pathol. 9, 143–151 (2006).
Choi, C. W. et al. Protective effect of chorioamnionitis on the development of bronchopulmonary dysplasia triggered by postnatal systemic inflammation in neonatal rats. Pediatr. Res. 79, 287–294 (2016).
Willet, K. E. et al. Antenatal endotoxin and glucocorticoid effects on lung morphometry in preterm lambs. Pediatr. Res. 48, 782–788 (2000).
Yee, M., Buczynski, B. W. & O’Reilly, M. A. Neonatal hyperoxia stimulates the expansion of alveolar epithelial type II cells. Am. J. Respir. Cell Mol. Biol. 50, 757–766 (2014).
Yee, M. et al. Type II epithelial cells are critical target for hyperoxia-mediated impairment of postnatal lung development. Am. J. Physiol. Lung Cell. Mol. Physiol. 291, L1101–L1111 (2006).
Deptula, N. et al. Brief mechanical ventilation causes differential epithelial repair along the airways of fetal, preterm lambs. Am. J. Physiol. Lung Cell. Mol. Physiol. 311, L412–L420 (2016).
Kemp, M. et al. The clinical use of corticosteroids in pregnancy. Hum. Reprod. Update 22, 240–259 (2015).
Curstedt, T., Halliday, H. L. & Speer, C. P. A unique story in neonatal research: the development of a porcine surfactant. Neonatology 107, 321–329 (2015).
Acknowledgements
We would like to thank Nico Kloosterboer and Lilian Kessels for their excellent technical assistance. This work was supported by a National Institutes of Health (Bethesda, MD, USA) grant (HD 57869) and by the Dutch Lung Foundation (Longfonds, Grant no. 6.1.16.088 to T.G.A.M.W., N.L.R., and P.G.J.N.).
Author information
Authors and Affiliations
Contributions
H.W., T.G.A.M.W., and N.L.R. contributed to the conception and design of the research; H.W., A.J.C.N.v.L., M.W.K., M.S.P., A.H.J., M.S., H.U., J.P.N., and B.W.K. participated in conduction of experiments, acquirement, and analysis of data; H.W., D.R.M.G.O., P.G.J.N., C.A.H.S.-R., V.L.S.L., B.W.K., T.D., T.G.A.M.W., and N.L.R. drafted, edited, and revised the manuscript. All the authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Patient consent
No patient consent was required for this study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Widowski, H., Ophelders, D.R.M.G., van Leeuwen, A.J.C.N. et al. Chorioamnionitis induces changes in ovine pulmonary endogenous epithelial stem/progenitor cells in utero. Pediatr Res 90, 549–558 (2021). https://doi.org/10.1038/s41390-020-01204-9
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41390-020-01204-9