Abstract
Background
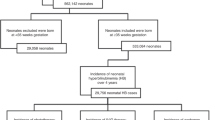
Hemolysis in fetus/newborns is often caused by maternal antibodies. There are currently no established screening procedures for maternal ABO antibodies harmful to fetus/newborn. We investigated the clinical significance, and predictive value of maternal anti-A/B titer for hyperbilirubinemia in ABO-incompatible newborns.
Methods
We conducted a case–control study of blood group O mothers and their ABO-compatible (O) vs. -incompatible (A/B) newborns receiving phototherapy, and of ABO-incompatible newborns receiving phototherapy vs. no phototherapy. Newborn data and treatment modalities were recorded, and total serum bilirubin and hemoglobin were measured. Maternal anti-A/B immunoglobulin-γ (IgG) titers were measured prenatally and perinatally, and negative and positive predictive values (NPV, PPV) were calculated to assess the risk of developing hyperbilirubinemia requiring phototherapy.
Results
We found a significantly higher maternal IgG antibody titer in the case group (p < 0.001). Maternal anti-A/B titers at first trimester had modest predictive values: NPV = 0.82 and PPV = 0.65 for neonatal hyperbilirubinemia; titers at birth improved the predictive values: NPV = 0.93 and PPV = 0.73. Newborn hemoglobin was significantly lower in incompatibles compared to compatibles (p = 0.034). Furthermore, increased anti-A/B IgG production during pregnancy was associated with hyperbilirubinemia and hemolysis in incompatible newborns.
Conclusions
There was a significant association between maternal anti-A/B IgG titer and hyperbilirubinemia requiring treatment.
Impact
-
Maternal anti-A/B IgG titer in the first trimester and at birth is predictive of hemolytic disease of the ABO-incompatible newborn.
-
Increased IgG anti-A/B production throughout pregnancy in mothers to ABO-incompatible newborns developing hyperbilirubinemia contrasts a constant or reduced production in mothers to newborns not developing hyperbilirubinemia.
-
Screening tools available in most immunohematology laboratories can identify clinically important IgG anti-A/B.
-
Use of maternal samples taken at birth yielded NPV = 0.93 and PPV = 0.73.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Le Pichon, J. B., Riordan, S. M., Watchko, J. & Shapiro, S. M. The neurological sequelae of neonatal hyperbilirubinemia: definitions, diagnosis and treatment of the kernicterus spectrum disorders (KSDs). Curr. Pediatr. Rev. 13, 199–209 (2017).
Donneborg, M. L., Vandborg, P. K., Hansen, B. M., Rodrigo-Domingo, M. & Ebbesen, F. Double versus single intensive phototherapy with LEDs in treatment of neonatal hyperbilirubinemia. J. Perinatol. 38, 154–158 (2018).
de Haas, M., Thurik, F. F., Koelewijn, J. M. & van der Schoot, C. E. Haemolytic disease of the fetus and newborn. Vox Sang. 109, 99–113 (2015).
Bakkeheim, E. et al. Maternal IgG anti-A and anti-B titres predict outcome in ABO-incompatibility in the neonate. Acta Paediatr. 98, 1896–1901 (2009).
Donneborg, M. L., Hansen, B. M., Vandborg, P. K., Rodrigo-Domingo, M. & Ebbesen, F. Extreme neonatal hyperbilirubinemia and kernicterus spectrum disorder in Denmark during the years 2000-2015. J. Perinatol. 40, 194–202 (2020).
Voak, D. & Bowley, C. C. A detailed serological study on the prediction and diagnosis of ABO haemolytic disease of the newborn (ABO HD). Vox Sang. 17, 321–348 (1969).
Kaplan, M., Hammerman, C., Vreman, H. J., Wong, R. J. & Stevenson, D. K. Hemolysis and hyperbilirubinemia in antiglobulin positive, direct ABO blood group heterospecific neonates. J. Pediatr. 157, 772–777 (2010).
Sarici, S. U. et al. An early (sixth-hour) serum bilirubin measurement is useful in predicting the development of significant hyperbilirubinemia and severe ABO hemolytic disease in a selective high-risk population of newborns with ABO incompatibility. Pediatrics 109, e53 (2002).
Chen, J. Y. & Ling, U. P. Prediction of the development of neonatal hyperbilirubinemia in ABO incompatibility. Zhonghua Yi Xue Za Zhi 53, 13–18 (1994).
Bratlid, D., Nakstad, B. & Hansen, T. W. National guidelines for treatment of jaundice in the newborn. Acta Paediatr. 100, 499–505 (2011).
Roback, J. D. Technical Manual 16th edn., 877–897 (American Association of Blood Banks, Bethesda, 2008).
Ching, E. Solid phase red cell adherence assay: a tubeless method for pretransfusion testing and other applications in transfusion science. Transfus. Apher. Sci. 46, 287–291 (2012).
Reverberi, R. The statistical analysis of immunohaematological data. Blood Transfus. 6, 37–45 (2008).
Youden, W. J. Index for rating diagnostic tests. Cancer 3, 32–35 (1950).
Zonneveld, R. et al. Severe fetal hemolysis and cholestasis due to high-titer maternal IgG anti-A antibodies. Pediatrics 143, e20182859 (2019).
Ziprin, J. H., Payne, E., Hamidi, L., Roberts, I. & Regan, F. ABO incompatibility due to immunoglobulin G anti-B antibodies presenting with severe fetal anaemia. Transfus. Med 15, 57–60 (2005).
McDonnell, M., Hannam, S. & Devane, S. P. Hydrops fetalis due to ABO incompatibility. Arch. Dis. Child Fetal Neonatal Ed. 78, F220–F221 (1998).
Preer, G. L. & Philipp, B. L. Understanding and managing breast milk jaundice. Arch. Dis. Child Fetal Neonatal Ed. 96, F461–F466 (2011).
Low, J. A., Johnston, E. E. & McBride, R. L. Blood volume adjustments in the normal obstetric patient with particular reference to the third trimester of pregnancy. Am. J. Obstet. Gynecol. 91, 356–363 (1965).
Malek, A., Sager, R., Kuhn, P., Nicolaides, K. H. & Schneider, H. Evolution of maternofetal transport of immunoglobulins during human pregnancy. Am. J. Reprod. Immunol. 36, 248–255 (1996).
Roh, E. Y. et al. Frequency of fetal-maternal microchimerism: an analysis of the HLA-DRB1 gene in cord blood and maternal sample pairs. J. Matern. Fetal Neonatal Med. 30, 2613–2619 (2017).
Jonsson, S. et al. Identification of sequence variants influencing immunoglobulin levels. Nat. Genet. 49, 1182–1191 (2017).
Cappellini, M. D. & Fiorelli, G. Glucose-6-phosphate dehydrogenase deficiency. Lancet 371, 64–74 (2008).
Nordvall, M. et al. Red blood cell antibodies in pregnancy and their clinical consequences: synergistic effects of multiple specificities. Transfusion 49, 2070–2075 (2009).
Acknowledgements
We thank laboratory technologists at the Serology Laboratory at the Department of Clinical Immunology, Rigshospitalet. We also thank the staff, social, and health care assistants, as well as midwives and nurses, at the maternity ward and the obstetric department at both Aalborg and Copenhagen University Hospital. Special thanks to the leading laboratory scientist, Jane Vad, for her invaluable help with blood grouping of newborns and handling of samples from Aalborg University Hospital. We would also like to thank the nurses and doctors at the including departments at Rigshospitalet, Herlev Hospital, Hvidovre Hospital, Nordsjællands Hospital, and Aalborg University Hospital for their help with the recruitment of patients.
Author information
Authors and Affiliations
Contributions
G.R.K. conceptualized and designed the study, collected the data, performed the statistical analyses, drafted the initial manuscript, and reviewed and revised the manuscript. M.L.D. conceptualized and designed the study, included patients, reviewed thoroughly the statistical analyses and clinical aspect, and revised the manuscript. B.M.H. conceptualized and designed the study, included patients, reviewed thoroughly the clinical aspect, and revised the manuscript. H.L. designed tables, reviewed thoroughly the statistical analyses, and reviewed and revised the manuscript. K.V.J., A.K.-T., P.A., and T.B. included patients, and reviewed and revised the manuscript. F.B.C., F.E., M.K.S., and M.H.D. conceptualized and designed the study, and reviewed and revised the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work. This work was supported by grants from Rigshospitalet’s and dbio’s Research Foundations.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Patient consent
Verbal and written consent was obtained from the mothers and the parents of the newborns.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Patient consent: Verbal and written consent was obtained from the mothers and the parents of the newborns.
Rights and permissions
About this article
Cite this article
Krog, G.R., Donneborg, M.L., Hansen, B.M. et al. Prediction of ABO hemolytic disease of the newborn using pre- and perinatal quantification of maternal anti-A/anti-B IgG titer. Pediatr Res 90, 74–81 (2021). https://doi.org/10.1038/s41390-020-01232-5
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-020-01232-5
This article is cited by
-
The timing of using IVIG for neonatal ABO hemolytic disease
BMC Pediatrics (2025)
-
End-tidal carbon monoxide concentrations measured within 48 hours of birth predict hemolytic hyperbilirubinemia
Journal of Perinatology (2024)
-
The Diagnostic Potential of the L Score for ABO Hemolytic Disease of the Newborn: Insights from a Cross-Sectional Study
Indian Journal of Hematology and Blood Transfusion (2024)