Abstract
Background
Potentially, orally administered antibodies specific to enteric pathogens could be administered to infants to prevent diarrheal infections, particularly in developing countries where diarrhea is a major problem. However, to prevent infection, such antibodies would need to resist degradation within the gastrointestinal tract.
Methods
Palivizumab, a recombinant antibody specific to respiratory syncytial virus (RSV), was used in this study as a model for examining the digestion of neutralizing antibodies to enteric pathogens in infants. The survival of this recombinant IgG1 across digestion in 11 infants was assayed via an anti-idiotype ELISA and RSV F protein-specific ELISA. Concentrations were controlled for any dilution or concentration that occurred in the digestive system using mass spectrometry-based quantification of co-administered, orally supplemented, indigestible polyethylene glycol (PEG-28).
Results
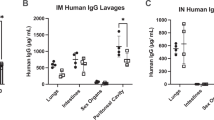
Binding activity of Palivizumab IgG1 decreased (26–99%) across each phase of in vivo digestion as measured by both anti-idiotype and RSV F protein-specific ELISAs.
Conclusion
Antibodies generated for passive protection of the infant gastrointestinal tract from pathogens will need to be more resistant to digestion than the model antibody fed to infants in this study, or provided in higher doses to be most effective.
Impact
-
Binding activity of palivizumab IgG1 decreased (26–99%) across each phase of in vivo infant digestion as measured by both anti-idiotype and RSV F protein-specific ELISAs.
-
Palivizumab was likely degraded by proteases and changes in pH introduced in the gut.
-
Antibodies generated for passive protection of the infant gastrointestinal tract from pathogens will need to be more resistant to digestion than the model antibody fed to infants in this study, or provided in higher doses to be most effective.
-
The monoclonal antibody IgG1 tested was not stable across the infant gastrointestinal tract.
-
The observation of palivizumab reduction was unlikely due to dilution in the gastrointestinal tract.
-
The results of this work hint that provision of antibody could be effective in preventing enteric pathogen infection in infants.
-
Orally delivered recombinant antibodies will need to either be dosed at high levels to compensate for digestive losses or be engineered to better resist digestion.
-
Provision of enteric pathogen-specific recombinant antibodies to at-risk infants could provide a new and previously unexplored pathway to reducing the infection in infants.
-
The strategy of enteric recombinant antibodies deserves more investigation throughout medicine as a novel means for treatment of enteric disease targets.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Kotloff, K. L. et al. The Global Enteric Multicenter Study (GEMS) of diarrheal disease in infants and young children in developing countries: epidemiologic and clinical methods of the case/control study. Clin. Infect. Dis. 55, S232–S245 (2012).
Walker, C. L. F., Aryee, M. J., Boschi-Pinto, C. & Black, R. E. Estimating diarrhea mortality among young children in low and middle income countries. PLoS ONE 7, e29151 (2012).
Lanata, C. F. et al. Global causes of diarrheal disease mortality in children <5 years of age: a systematic review. PLoS ONE 8, e72788 (2013).
Walker, R. I. Considerations for development of whole cell bacterial vaccines to prevent diarrheal diseases in children in developing countries. Vaccine 23, 3369–3385 (2005).
Hanson, L. A. Breastfeeding provides passive and likely long-lasting active immunity. Ann. Allergy Asthma Immunol. 81, 523–533 (1998).
Wu, H., Pfarr, D. S., Losonsky, G. A. & Kiener, P. A. Immunoprophylaxis of RSV infection: advancing from RSV-IGIV to palivizumab and motavizumab. Curr. Top. Microbiol. Immunol. 317, 103–123 (2008).
Lueangsakulthai, J., Sah, B. N. P., Scottoline, B. P. & Dallas, D. C. Survival of recombinant monoclonal and naturally-occurring human milk immunoglobulins A and G specific to respiratory syncytial virus F protein across simulated human infant gastrointestinal digestion. J. Funct. Foods 73, 104115 (2020).
Lueangsakulthai, J., Sah, B. N. P., Scottoline, B. P. & Dallas, D. C. Survival of recombinant monoclonal antibodies (IgG, IgA and sIgA) versus naturally-occurring antibodies (IgG and sIgA/IgA) in an ex vivo infant digestion model. Nutrients. 12 (2020).
Pelegri-O’Day, E. M., Lin, E.-W. & Maynard, H. D. Therapeutic protein-polymer conjugates: advancing beyond PEGylation. J. Am. Chem. Soc. 136, 14323–14332 (2014).
Brady, C. E. 3rd et al. Urinary excretion of polyethylene glycol 3350 and sulfate after gut lavage with a polyethylene glycol electrolyte lavage solution. Gastroenterology 90, 1914–1918 (1986).
Demers-Mathieu, V., Lueangsakulthai, J., Qu, Y., Scottoline, B. P. & Dallas, D. C. Binding and neutralizing capacity of respiratory syncytial virus (RSV)-specific recombinant IgG against RSV in human milk, gastric and intestinal fluids from infants. Nutrients 12 (2020).
Kim, B. J., Lueangsakulthai, J., Sah, B. N. P., Scottoline, B. & Dallas, D. C. Quantitative analysis of antibody survival across the infant digestive tract using mass spectrometry with parallel reaction monitoring. Foods 759 (2020).
Kollmann, T. R., Kampmann, B., Mazmanian, S. K., Marchant, A. & Levy, O. Protecting the newborn and young infant from infectious diseases: lessons from immune ontogeny. Immunity 46, 350–363 (2017).
Arnold, B. F. et al. Cluster-randomised controlled trials of individual and combined water, sanitation, hygiene and nutritional interventions in rural Bangladesh and Kenya: the WASH Benefits study design and rationale. BMJ Open 3, e003476 (2013).
Humphrey, J. H. et al. The sanitation hygiene infant nutrition efficacy (SHINE) trial: rationale, design, and methods. Clin. Infect. Dis. 61, S685–S702 (2015).
Dewey, K. G. & Begum, K. Long-term consequences of stunting in early life. Matern. Child Nutr. 7, 5–18 (2011).
Casale, D., Desmond, C. & Richter, L. The association between stunting and psychosocial development among preschool children: a study using the South African Birth to Twenty cohort data. Child Care Health Dev. 40, 900–910 (2014).
American Academy of Pediatrics. Breastfeeding and maternal and infant health outcomes in developed countries. AAP Gd. Rounds 18, 15–16 (2007).
Victoria, C. G. Effect of breastfeeding on infant and child mortality due to infectious diseases in less developed countries: a pooled analysis. Lancet 355, 451–455 (2000).
Labbok, M. H., Clark, D. & Goldman, A. S. Breastfeeding: maintaining an irreplaceable immunological resource. Nat. Rev. Immunol. 4, 565–572 (2004).
Lanari, M. et al. Maternal milk protects infants against bronchiolitis during the first year of life. Results from an Italian cohort of newborns. Early Hum. Dev. 89, S51–S57 (2013).
Boone, K. M., Geraghty, S. R. & Keim, S. A. Feeding at the breast and expressed milk feeding: associations with otitis media and diarrhea in infants. J. Pediatr. 174, 118–125 (2016).
Ruiz-Palacios, G. M. et al. Protection of breast-fed infants against Campylobacter diarrhea by antibodies in human milk. J. Pediatr. 116, 707–713 (1990).
Glass, R. I. & Stoll, B. J. The protective effect of human milk against diarrhea: a review of studies from Bangladesh. Acta Paediatr. 78, 131–136 (1989).
Robbie, G. J., Zhao, L., Mondick, J., Losonskym, G. & Roskos, L. K. Population pharmacokinetics of palivizumab, a humanized anti-respiratory syncytial virus monoclonal antibody, in adults and children. Antimicrob. Agents Chemother. 56, 4927–4936 (2012).
Lamoyi, E. & Nisonoff, A. Preparation of F(ab′)2 fragments from mouse IgG of various subclasses. J. Immunol. Methods 56, 235–243 (1983).
Svasti, J. & Milstein, C. The disulphide bridges of a mouse immunoglobulin G1 protein. Biochem. J. 126, 837–850 (1972).
Mason, D. W. & Williams, A. F. The kinetics of antibody binding to membrane antigens in solution and at the cell surface. Biochem. J. 187, 1–20 (1980).
Parham, P. On the fragmentation of monoclonal IgG1, IgG2a, and IgG2b from BALB/c mice. J. Immunol. 131, 2895–2902 (1983).
Mariant, M., Camagna, M., Tarditi, L. & Seccamani, E. A new enzymatic method to obtain high-yield F(ab)2 suitable for clinical use from mouse IgGl. Mol. Immunol. 28, 69–77 (1991).
Rao, P. E. & Kroon, D. J. Orthoclone OKT3. Chemical mechanisms and functional effects of degradation of a therapeutic monoclonal antibody. Pharm. Biotechnol. 5, 135–158 (1993).
Hiatt, A. et al. Glycan variants of a respiratory syncytial virus antibody with enhanced effector function and in vivo efficacy. Proc. Natl Acad. Sci. USA 111, 5992–5997 (2014).
Jefferis, R. Glycosylation as a strategy to improve antibody-based therapeutics. Nat. Rev. Drug Discov. 8, 226–234 (2009).
Sah, B. N. P. et al. Partial degradation of recombinant antibody functional activity during infant gastrointestinal digestion: implications for oral antibody supplementation. Front. Nutr. 7 (2020).
Acknowledgements
The authors thank Matt Paluska, Anahi Y. Torres-Pimentel, Jooyoung Yeo, Siana Liti, and Kimberly R. Lane for their assistance with the ELISA experiments. This study was supported by the Bill & Melinda Gates Foundation (OPP1183649). The authors also acknowledge support from the OSU Mass Spectrometry Center in part through instrumentation grant NIH # 1S10OD020111-01 and the Oregon State University Research Office.
Author information
Authors and Affiliations
Contributions
J.L., B.J.K., V.D.-M., B.N.P.S., Y.W., A.O., M.A., A.O'C., B.P.S., and D.C.D conceptualized the study. J.L. and B.J.K designed the experiments, performed data acquisition, and analyzed data. J.L. drafted the article. D.C.D supervised the experiments. All authors revised, adapted, and approved final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Patient consent
Patient consent was required for this basic science study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Lueangsakulthai, J., Kim, B.J., Demers-Mathieu, V. et al. Effect of digestion on stability of palivizumab IgG1 in the infant gastrointestinal tract. Pediatr Res 90, 335–340 (2021). https://doi.org/10.1038/s41390-020-01271-y
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41390-020-01271-y