Abstract
Background
Perinatal inflammation adversely affects health. Therefore, aims of this IRB-approved study are: (1) compare inflammatory compounds within and between maternal and umbilical cord blood samples at the time of delivery, (2) assess relationships between inflammatory compounds in maternal and cord blood with birth characteristics/outcomes, and (3) assess relationships between blood and placental fat-soluble nutrients with blood levels of individual inflammatory compounds.
Methods
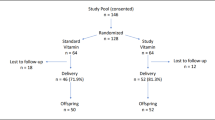
Mother−infant dyads were enrolled (n = 152) for collection of birth data and biological samples of maternal blood, umbilical cord blood, and placental tissue. Nutrient levels included: lutein + zeaxanthin; lycopene; α-, β-carotene; β-cryptoxanthin; retinol; α-, γ-, δ-tocopherol. Inflammatory compounds included: tumor necrosis factor-α, superoxide dismutase, interleukins (IL) 1β, 2, 6, 8, 10.
Results
Median inflammatory compound levels were 1.2–2.3 times higher in cord vs. maternal blood, except IL2 (1.3 times lower). Multiple significant correlations existed between maternal vs. infant inflammatory compounds (range of r = 0.22–0.48). While relationships existed with blood nutrient levels, the most significant were identified in placenta where all nutrients (except δ-tocopherol) exhibited relationships with inflammatory compounds. Relationships between anti-inflammatory nutrients and proinflammatory compounds were primarily inverse.
Conclusion
Inflammation is strongly correlated between mother−infant dyads. Fat-soluble nutrients have relationships with inflammatory compounds, suggesting nutrition is a modifiable factor.
Impact
-
Mother and newborn inflammation status are strongly interrelated.
-
Levels of fat-soluble nutrients in blood, but especially placenta, are associated with blood levels of proinflammatory and anti-inflammatory compounds in both mother and newborn infant.
-
As fat-soluble nutrient levels are associated with blood inflammatory compounds, nutrition is a modifiable factor to modulate inflammation and improve perinatal outcomes.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Armstrong-Wells, J. et al. Inflammatory predictors of neurologic disability after preterm premature rupture of membranes. Am. J. Obstet. Gynecol. 212(Feb), 212.e1–212.e9 (2015).
Bi, D. et al. The association between sex-related interleukin-6 gene polymorphisms and the risk for cerebral palsy. J. Neuroinflammation. 11(Jun), 100 (2014).
Gilman, S. E. et al. Prenatal immune programming of the sex-dependent risk for major depression. Transl. Psychiatry 6(May), e822 (2016).
Brown, A. S. et al. Elevated maternal interleukin-8 levels and risk of schizophrenia in adult offspring. Am. J. Psychiatry 161(May), 889–895 (2004).
Buka, S. L. et al. Maternal cytokine levels during pregnancy and adult psychosis. Brain Behav. Immun. 15(Dec), 411–420 (2001).
Tian, C., Cheng, L. & Gu, X. Cord blood TNF-alpha and IL-6 levels as diagnostic indicators of brain damage in neonates with non-asphyxia fetal distress. Arch. Gynecol. Obstet. 295(Feb), 337–342 (2017).
Rasmussen, J. M. et al. Maternal interleukin-6 concentration during pregnancy is associated with variation in frontolimbic white matter and cognitive development in early life. Neuroimage 185(Jan), 825–835 (2019).
Blanco-Quiros, A. et al. Cord blood interleukin-10 levels are increased in preterm newborns. Eur. J. Pediatr. 159(Jun), 420–423 (2000).
Rose, J., Vassar, R., Cahill-Rowley, K., Hintz, S. R. & Stevenson, D. K. Neonatal biomarkers of inflammation: correlates of early neurodevelopment and gait in very-low-birth-weight preterm children. Am. J. Perinatol. 33(Jan), 71–78 (2016).
Cuestas, E., Aguilera, B., Cerutti, M. & Rizzotti, A. Sustained neonatal inflammation is associated with poor growth in infants born very preterm during the first year of life. J. Pediatr. 205(Feb), 91–97 (2019).
Ramel, S. E. et al. The relationship of poor linear growth velocity with neonatal illness and two-year neurodevelopment in preterm infants. Neonatology 102, 19–24 (2012).
Shim, Y. J. et al. Inflammatory and immune proteins in umbilical cord blood: association with hearing screening test failure in preterm neonates. Mediators Inflamm. 2018(Sep), 4209359 (2018).
Trevino-Garza, C. et al. Leptin, IL-6 and TNF-alpha levels in umbilical cord blood of healthy term newborns in relation to mode of delivery. J. Obstet. Gynaecol. 36(Aug), 719–721 (2016).
Gedikbasi, A. et al. The evaluation of cord blood interleukin-1beta levels in normal and caesarean deliveries. Hum. Exp. Toxicol. 33(Dec), 1193–1198 (2014).
Wampach, L. et al. Birth mode is associated with earliest strain-conferred gut microbiome functions and immunostimulatory potential. Nat. Commun. 30(Nov), 5091 (2018).
Hu, Y. et al. Placenta response of inflammation and oxidative stress in low-risk term childbirth: the implication of delivery mode. BMC Pregnancy Childbirth 17(Dec), 407 (2017).
Tutdibi, E., Hunecke, A., Lindner, U., Monz, D. & Gortner, L. Levels of cytokines in umbilical cord blood in relation to spontaneous term labor. J. Perinat. Med. 40(Sep), 527–532 (2012).
Cusick, S. E. & Georgieff, M. K. The Role of Nutrition in Brain Development: The Golden Opportunity of the “First 1000 Days”. J Pediatr. 175, 16–21. https://doi.org/10.1016/j.jpeds.2016.05.013 (2016).
Institute of Medicine (US) Panel on Micronutrients. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc (National Academy of Sciences, Washington, DC, 2001).
Institute of Medicine (US) Panel on Dietary Antioxidants and Related Compounds. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids (National Academies Press, Washington, DC, 2000).
Shah, M. D. & Shah, S. R. Nutrient deficiencies in the premature infant. Pediatr. Clin. North Am. 56(Oct), 1069–1083 (2009).
Hanson, C., Lyden, E., Furtado, J., Van Ormer, M. & Anderson-Berry, A. A. Comparison of nutritional antioxidant content in breast milk, donor milk, and infant formulas. Nutrients 8(Oct), E681 (2016).
Ozsurekci, Y. & Aykac, K. Oxidative stress related diseases in newborns. Oxid. Med. Cell Longev. 2016, 2768365 (2016).
United States Department of Agriculture. USDA Food Composition Databases https://ndb.nal.usda.gov/ndb/nutrients/. Accessed July 31, 2020.
World Health Organization. Preterm Birth. 2018. https://www.who.int/news-room/fact-sheets/detail/preterm-birth. Accessed July 31, 2020.
Zhou, Z. W. et al. Erythropoietin regulates immune/inflammatory reaction and improves neurological function outcomes in traumatic brain injury. Brain Behav. 7(Oct), e00827 (2017).
Machova Urdzikova, L. et al. A green tea polyphenol epigallocatechin-3-gallate enhances neuroregeneration after spinal cord injury by altering levels of inflammatory cytokines. Neuropharmacology 126(Nov), 213–223 (2017).
Yahfoufi, N., Alsadi, N., Jambi, M., & Matar, C. The Immunomodulatory and anti-inflammatory role of polyphenols. Nutrients https://doi.org/10.3390/nu10111618 (2018).
Dojindo Molecular Technologies, I. SOD Assay Kit-WST. https://www.dojindo.com/product/sod-assay-kit-wst-s311/. Accessed November 30, 2020.
El-Sohemy, A. et al. Individual carotenoid concentrations in adipose tissue and plasma as biomarkers of dietary intake. Am. J. Clin. Nutr. 76(Jul), 172–179 (2002).
Sorokin, Y. et al. Maternal serum interleukin-6, C-reactive protein, and matrix metalloproteinase-9 concentrations as risk factors for preterm birth. Am J Neonatol. 8(Sept), 631–640 (2010). https://doi.org/10.1055/s-0030-1249366.
An, H. et al. Interleukin-6, interleukin-8, and soluble tumor necrosis factor receptor-I in the cord blood as predictors of chronic lung disease in premature infants. Am. J. Obstet. Gynecol. 191(Nov), 1649–1654 (2004).
Zielinska, M. A., Wesolowska, A., Pawlus, B. & Hamulka, J. Health Effects of carotenoids during pregnancy and lactation. Nutrients https://doi.org/10.3390/nu9080838 (2017).
Acknowledgements
Carotenoid analysis was funded by the Child Health Research Institute by Children’s Hospital & Medical Center and the University of Nebraska Medical Center. Inflammatory mediator analysis was funded by the University of Nebraska Medical Center Clinical Translational Research Mentored Scholars Program Pilot Award to J.N.S.
Author information
Authors and Affiliations
Contributions
Conceptualization, all authors; methodology, all authors; software, all authors; validation, all authors; formal analysis, E.R.L.; investigation, all authors; resources, all authors; data curation, all authors; investigation, all authors; writing—original draft preparation, M.K.T. and J.N.S.; writing—review and editing, all authors; visualization, all authors; supervision; A.L.A.-B., C.K.H. and J.N.S.; project administration, J.N.S.; funding acquisition, M.K.T., A.L.A.-B., C.K.H. and J.N.S.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent statement
Written informed consent was obtained for each mother−infant dyad before enrollment into this study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Thoene, M.K., Van Ormer, M.C., Lyden, E.R. et al. Concentrations of fat-soluble nutrients and blood inflammatory compounds in mother−infant dyads at birth. Pediatr Res 90, 436–443 (2021). https://doi.org/10.1038/s41390-020-01302-8
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41390-020-01302-8