Abstract
Background
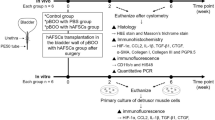
This study investigated whole neurogenic bladder’s progression changes, as well as the expression of TGF-β1 fibrosis pathway-related proteins in bilateral spinal nerve-amputated juvenile rats.
Methods
Sixty-four 8-week-old rats (32 bilateral L6 + S1 spinal nerve amputated and 32 sham operated) were selected. Cystometry was performed. General assessments, Masson, Sirius red, immunohistochemical staining, and western blotting of fibrosis and TGF-β1 pathway-related proteins were conducted using bladder tissues.
Results
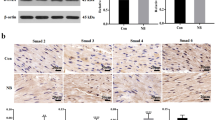
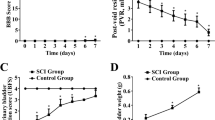
Cystometry results showed that the basal intravesical pressures and bladder capacities in nerve-amputated rats were significantly higher than those in sham-operated ones. Compared to the sham-operated groups, the bladder size and wall thickness in the nerve-amputated groups increased initially but then decreased over time. However, bladder weight continuously increased over time. Disintegration, thickening, and hypertrophy of the bladder wall were found over time in the amputated rats. Moreover, there was a significant increase in collagen III, and the ratio of collagen III/I was higher in amputated rats (P < 0.01). Finally, the expression of TGF-β1, TGF-βRI, Smad2, and collagen III and I increased in amputated bladder tissues, while Smad6 decreased over time.
Conclusions
The main clinical features of pediatric neurogenic bladder (PNB) were detrusor paralysis and continuous intravesical pressure. Biological molecular findings are earlier than the pathophysiological findings. Therefore, early preventing bladder fibrosis by targeting TGF-β1/Smad pathway-related proteins once knowing the PNB diagnosis might be an alternative treatment for PNB.
Impact
-
The study found that the main clinical features of PNB were detrusor paralysis, continuous intravesical pressure, and increased TGF-beta/Smad signal proteins over time.
-
The study makes contributions to the literature because it suggests biological molecular findings are earlier than the pathophysiological findings by various staining in PNB.
-
The study investigated whole neurogenic bladder’s progression changes, as well as the expression of TGF-β1 fibrosis pathway-related proteins in the spinal nerve-injured PNB juvenile rat models, which suggests that early prevention of bladder fibrosis by targeting TGF-β1/Smad pathway-related proteins once knowing the PNB diagnosis might be an alternative treatment for pediatric neurogenic bladder.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Amarenco, G., Sheikh Ismaël, S., Chesnel, C., Charlanes, A. & Breton, L. E. F. Diagnosis and clinical evaluation of neurogenic bladder. Eur. J. Phys. Rehabil. Med. 53, 975–980 (2017).
Sripathi, V. & Mitra, A. Management of neurogenic bladder. Indian J. Pediatr. 84, 545–554 (2017).
Johnston, A. W., Wiener, J. S. & Todd Purves, J. Pediatric neurogenic bladder and bowel dysfunction: will my child ever be out of diapers? Eur. Urol. Focus 6, 838–867 (2020).
Huen, K. H., Nik-Ahd, F., Chen, L., Lerman, S. & Singer, J. Neomycin-polymyxin or gentamicin bladder instillations decrease symptomatic urinary tract infections in neurogenic bladder patients on clean intermittent catheterization. J. Pediatr. Urol. 15, 178.e1–178.e7 (2019).
Stein, R. et al. EAU/ESPU guidelines on the management of neurogenic bladder in children and adolescent part I diagnostics and conservative treatment. Neurourol. Urodyn. 39, 45–57 (2020).
Tuite, G. F. et al. Lack of efficacy of an intradural somatic-to-autonomic nerve anastomosis (Xiao procedure) for bladder control in children with myelomeningocele and lipomyelomeningocele: results of a prospective, randomized, double-blind study. J. Neurosurg. Pediatr. 18, 150–163 (2016).
de Barros, R. S. M. et al. Morphofunctional evaluation of end-to-side neurorrhaphy through video system magnification. J. Surg. Res. 221, 64–68 (2018).
Gao, W., Liu, Q., Li, S., Zhang, J. & Li, Y. End-to-side neurorrhaphy for nerve repair and function rehabilitation. J. Surg. Res. 197, 427–435 (2015).
Lai, J. et al. Activation of NFKB-JMJD3 signaling promotes bladder fibrosis via boosting bladder smooth muscle cell proliferation and collagen accumulation. Biochim. Biophys. Acta. 1865, 2403–2410 (2019).
Li, Y. L. et al. Reconstruction of bladder function and prevention of renal deterioration by means of end-to-side neurorrhaphy in rats with neurogenic bladder. Neurourol. Urodyn. 37, 1272–1280 (2018).
Tohru, T., Kazuki, U. & Akiyoshi, K. End-to-end and end-to-side neurorrhaphy between thick donor nerves and thin recipient nerves: an axon regeneration study in a rat model. Neural Regen. Res. 13, 699–703 (2018).
Naqvi, S., Clothier, J., Wright, A. & Garriboli, M. Urodynamic outcomes in children after single and multiple injections for overactive and low compliance neurogenic bladder treated with abobotulinum toxin A. J. Urol. 203, 413–419 (2020).
Doyle, C. et al. The role of the mucosa in modulation of evoked responses in the spinal cord injured rat bladder. Neurourol. Urodyn. 37, 1583–1593 (2018).
Liu, Q., Wang, R., Wang, C., Li, Y. & Li, A. The protective role of Schwann cells in bladder smooth muscle cell fibrosis. Int. J. Clin. Exp. Pathol. 12, 3799–3806 (2019).
Lu, Y. T., Tingskov, S. J., Djurhuus, J. C., Nørregaard, R. & Olsen, L. H. Can bladder fibrosis in congenital urinary tract obstruction be reversed? J. Pediatr. Urol. 13, 574–580 (2017).
Şekerci, Ç. A. et al. Urinary NGF, TGF-beta1, TIMP-2 and bladder wall thickness predict neurourological findings in children with myelodysplasia. J. Urol. 191, 199–205 (2014).
Zou, L. X. et al. Resveratrol attenuates pressure overload-induced cardiac fibrosis and diastolic dysfunction via PTEN/AKT/Smad2/3 and NF-kappaB signaling pathways. Mol. Nutr. Food Res. 63, e1900418 (2019).
Tang, W. B. et al. Effect of pressure on liver stiffness during the development of liver fibrosis in rabbits. Ultrasound Med. Biol. 42, 282–289 (2016).
Huang, Y. et al. Collagen type VI alpha 3 chain promotes epithelial-mesenchymal transition in bladder cancer cells via transforming growth factor beta (TGF-beta)/Smad pathway. Med. Sci. Monit. 24, 5346–5354 (2018).
Liang, R., Fisher, M., Yang, G., Hall, C. & Woo, S. L. Alpha1,3-galactosyltransferase knockout does not alter the properties of porcine extracellular matrix bioscaffolds. Acta Biomater. 7, 1719–1727 (2011).
Jiang, X. et al. Sodium tanshinone IIA sulfonate ameliorates bladder fibrosis in a rat model of partial bladder outlet obstruction by inhibiting the TGF-β/Smad pathway activation. PLoS ONE 10, e0129655 (2015).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant numbers: U1904208 and 81670689). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
Y.C. and Y.M. conceived and designed the experiments, performed the experiments, analyzed the data, wrote the paper, prepared figures, reviewed drafts of the paper, redid the experiments, and revised the paper. Y.H., D.X., and E.L. repeated the experiments, analyzed the data, and reviewed drafts of the paper. X.Y. and Y.H. performed the experiments and reviewed drafts of the paper. W.Z. and Q.W. contributed materials and reviewed drafts of the paper. J.G.W. conceived and designed the experiments, got the financial supports from the Foundations, provided the experimental platform and equipment, and reviewed drafts of the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Patient consent
Patient consent was not required.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Chen, Y., Ma, Y., He, Y. et al. The TGF-β1 pathway is early involved in neurogenic bladder fibrosis of juvenile rats. Pediatr Res 90, 759–767 (2021). https://doi.org/10.1038/s41390-020-01329-x
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-020-01329-x
This article is cited by
-
The Overactive and Poorly Compliant Bladder: a Review of Coexisting Detrusor Overactivity and Poor Compliance
Current Bladder Dysfunction Reports (2025)
-
Activation of the TGF-β1/Smads/α-SMA pathway is related to histological and functional changes in children with neurogenic bladder
Scientific Reports (2022)