Abstract
Background
The diagnosis of IBD and evaluation of treatment require endoscopy, which is difficult in children. This study evaluated the use of TFF3 as a biomarker.
Methods
Permeability of the intestinal mucosa and serum TFF3 were assayed and colon tissue was harvested 7 days after inducing IBD in mice with TNBSA. TFF3 was monitored in 51 pediatric IBD patients stratified by active disease or remission and in 20 healthy children. Mucosal healing was assessed by the Simple Endoscopic Score for Crohn Disease and Baron scores in CD and UC patients.
Results
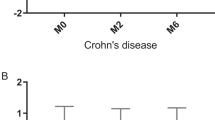
Histological evaluation revealed transmural inflammation of the colon in IBD model mice. Permeability of the intestinal mucosa and serum TFF3 were both higher in TNBSA-treated than in control mice (P < 0.05). TFF3 was higher in children with active IBD than in those in remission and in healthy children (P < 0.05). TFF3 was positively correlated with the SES-CD score (P < 0.05) but not with either the pediatric CDAI score or the serum CRP. The sensitivity of serum TFF3 for monitoring CD activity was 100% and the specificity was 76.2%.
Conclusions
TFF3 level increased with CD activity, which is of significance for diagnosis and for evaluation of mucosal healing. TFF3 could also be a marker in pediatric UC, as TFF3 positively correlated with UCAI.
Impact
-
The diagnosis and evaluation of IBD is difficult; endoscopy provides objective assessment; TFF3 can be a useful marker instead of endoscopy.
-
TFF3 was increased in active CD of children; TFF3 can be used as a clinical marker of pediatric CD; TFF3 can diagnose and evaluate mucosal healing of CD.
-
Pediatrician should pay attention to clinical marker; TFF3 level may be a key evaluation of mucosal healing of CD; the value of diagnosis of TFF3 in CD is important.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Ng, S. C. et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet 390, 2769–2778 (2018).
Guan, D. X. et al. Single center retrospective study of 184 children with inflammatory bowel disease seen from 2000–2014. Zhonghua Er Ke Za Zhi. 55, 493–498 (2017).
Teng, X., Xu, L. F., Sun, M. & Guo, j Phenotypic characteristics and clinical manifestations of inflammatory bowel disease in infants and children under 2 years of age in Liaoning Province, China: five of six infants with IL-10R mutations[J]. Paediatr. Int. Child Health 39, 59–64 (2019).
Ebrahimi, S. M. S. et al. Neonatal presentation of unremitting inflammatory bowel disease. Iran. J. Med. Sci. 43, 328–331 (2018).
Dinwiddie, D. L. et al. Molecular diagnosis of infantile onset inflammatory bowel disease by exome sequencing. Genomics 102, 442–447 (2013).
Al-Qabandi, W. A. et al. Inflammatory bowel disease in children, an evolving problem in Kuwait. Saudi J. Gastroenterol. 17, 323–327 (2011).
Fang, Y. H., Luo, Y. Y., Yu, J. D., Lou, J. G. & Chen, J. Phenotypic and genotypic characterization of inflammatory bowel disease in children under six years of age in China. World J. Gastroenterol. 24, 1035–1045 (2018).
Peng, K. et al. Blood transplantation corrects very early-onset inflammatory bowel disease in Chinese patients with IL10RA-associated immune deficiency. Inflamm. Bowel Dis. 24, 1416–1427 (2018).
Ye, Z. et al. Phenotype and management of infantile-onset inflammatory bowel disease: experience from a tertiary care center in China. Inflamm. Bowel Dis. 23, 2154–2164 (2017).
Sheikh, S., Uno, J., Matsuoka, K. & Plevy, S. Abnormal mucosal immune response to altered bacterial flora following restorative proctocolectomy in patients with ulcerative colitis: serologic measures, immunogenetics, and clinical correlations. Clin. Immunol. 127, 270–279 (2008).
Aschard, H. et al. Genetic effects on the commensal microbiota in inflammatory bowel disease patients. PLoS Genet. 15, e1008018 (2019).
Pei, L. Y. et al. Role of colonic microbiota in the pathogenesis of ulcerative colitis. BMC Gastroenterol. 19, 10 (2019).
Shen, B. The evaluation of postoperative patients with ulcerative colitis. Gastrointest. Endosc. Clin. N. Am. 26, 669–677 (2016).
Ylisaukko-Oja, T. et al. High treatment persistence rate and significant endoscopic healing among real-life patients treated with vedolizumab—a Finnish Nationwide Inflammatory Bowel Disease Cohort Study (FINVEDO). Scand. J. Gastroenterol. 53, 158–167 (2018).
Froslie, K. F., Jahnsen, J., Moum, B. A. & Vatn, M. H., IBSEN Group. Mucosal healing in inflammatory bowel disease: results from a Norwegian population-based cohort[J]. Gastroenterology 133, 412–422 (2007).
Rutgeerts, P., Vermeire, S. & Van Assche, G. Mucosal healing in inflammatory bowel disease: impossible ideal or therapeutic target?. Gut 56, 453–455 (2007).
Wu, S. N., Wu, J. & Hang, Y. Clinical utility of capsule endoscopy in 145 pediatric patients[J]. Shanghai Med. J. 5, 275–279 (2017).
Turner, D. et al. Appraisal of the pediatric ulcerative colitis activity index (PUCAI). Inflamm. Bowel Dis. 15, 1218–1223 (2010).
van Rheenen, P. F., de Vijver, E. V. & Fidler, V. Faecal calprotectin for screening of patients with suspected inflammatory bowel disease:diagnostic meta-analysis. BMJ 341, c3369 (2010).
Smith, L. A. & Gaya, D. R. Utility of faecal calprotectin analysis in adult inflammatory bowel disease. World J. Gastroenterol. 18, 6782–6789 (2012).
Ayling, R. M. & Kok, K. Fecal calprotectin. Adv. Clin. Chem. 87, 161–190 (2018).
Thim, L. Trefoil peptides: from structure to function. Cell. Mol. Life Sci. 53, 888–903 (1997).
Renes, I. B. et al. Distict epithelial responses in experimental colitis: implications for ion uptake and mucosal protection. Am. J. Physiol. Gastrointest. Liver Physiol. 283, G169–G179 (2002).
Chen, L. P., Zhang, B. H., Li, Y. & Chen, Z. The effect and significance of intestinal trefoil factor IL-8 and MDA for neonatal rat model for hypoxia-induced intestinal injury. Chin. J. Perinat. Med. 36, 306–309 (2003).
Li, J. et al. Protective effects of recombinant intestinal trefoil factor against intestinal injuries induced by endotoxin in young rats. Chin. J. Contemp. Pediatrics 8, 425–428 (2006).
Teng, X., Xu, L. F., Zhou, P., Sun, H. W. & Sun, M. Effects of trefoil peptide 3 on expression of TNF-α, TLR4,and NF-κB in trinitrobenzene sulphonic acid induced colitis mice. Inflammation 32, 120–129 (2009).
Srivastava, S. et al. Serum human trefoil factor 3 is a biomarker for mucosal healing in ulcerative colitis patients with minimal disease activity. J. Crohns Colitis 9, 575–579 (2015).
Stallmach, A. et al. An interleukin 12 p40-IgG2b fusion protein abrogates T cell mediated inflammation: anti-inflammatory activity in Cohn’s disease and experimental colitis in vivo. Gut 53, 339–345 (2004).
Chen, J. et al. Consensus on diagnostic norms for pediatric inflammatory bowel disease. Chin. J. Pract. Pediatrics 25, 263–265 (2010).
Turner, D. et al. Development, validation, and evaluation of a pediatric ulcerative colitis activity index: a prospective multicenter study. Gastroenterology 133, 423–432 (2007).
Hyams, J. S. et al. Development and validation of a pediatric Crohn’s disease activity index. J. Pediatr. Gastroenterol. Nutr. 12, 439–447 (1991).
Daperno, M. et al. Development and validation of a new, simplified endoscopic activity score for Crohn’s disease: the SES-CD. Gastrointest. Endosc. 60, 505–512 (2004).
Baron, J. H., Connell, A. M. & Lennard-Jones, J. Variation between observers in describing mucosal appearances in proctocolitis. Br. Med. J. 1, 89–92 (1964).
Acknowledgements
This work was supported by the National Natural Science Foundation of China Youth Science Foundation Project (no. 81400585) and Natural Science Foundation of Liaoning Provence, China (grant no. 2019-MS-372).
Author information
Authors and Affiliations
Contributions
X.T. had the primary responsibility for protocol development, outcome assessment, preliminary data analysis and drafting the manuscript. L.L., Y.Y. and L.Y. participated in the development of the protocol, animal experiments, collecting medical records of patient screening and analytical framework for the study and contributed to the writing of the manuscript. L.X., M.S. and J.W. supervised the design and execution of the study and revised the article critically for important intellectual content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Teng, X., Yang, Y., Liu, L. et al. Evaluation of inflammatory bowel disease activity in children using serum trefoil factor peptide. Pediatr Res 88, 792–795 (2020). https://doi.org/10.1038/s41390-020-0812-y
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41390-020-0812-y