Abstract
Background
There is limited information on gut microbiota of neonates with congenital gastrointestinal surgical conditions (CGISCs) available.
Methods
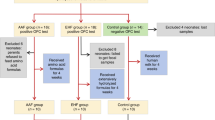
This study compared stool microbiota and short-chain fatty acids (SCFAs) of 37 term infants with CGISCs with 36 term healthy infants (HIs). Two stool samples were collected from each infant: as soon as possible after birth (week 1) and 10–14 days of life (week 2).
Results
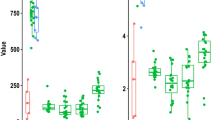
Bacterial richness and alpha diversity were comparable between CGISCs and HIs at week 1 and week 2 (all p > 0.05). Beta diversity analysis revealed that at week 1, CGISCs had similar community structures to HIs (p = 0.415). However, by week 2, community structures of CGISCs were significantly different from HIs (p = 0.003). At week 1, there were no significant differences in the relative abundances of genera Bifidobacterium and Bacteroides between CGISCs and HIs. At week 2, the relative abundance of Bifidobacterium was significantly lower in CGISCs (mean percentage 7.21 ± 13.49 vs. 28.96 ± 19.6; p = 0.002). Bacteroides were also less abundant in the CGISC group (mean percentage 0.12 ± 0.49 vs. 6.59 ± 8.62; p = 0.039). Relative abundance of genera Pseudomonas and Escherichia–Shigella were higher in CGISCs. At week 2, stool concentrations of all SCFAs were lower in CGISCs (all p < 0.001).
Conclusions
During hospitalization, neonates with CGISCs develop gut dysbiosis and deficiency of SCFAs.
Impact
-
During hospitalisation, neonates with congenital gastrointestinal surgical conditions develop gut dysbiosis with deficiency of Bifidobacteria and Bacteroides and increased abundance of Escherichia-Shigella and Pseudomonas. They also have low levels of short chain fatty acids in their stools compared to healthy infants.
-
This is the first study evaluating the gut microbiota using 16S ribosomal RNA sequencing methods and stool short chain fatty acids in neonates with congenital gastrointestinal surgical conditions and comparing them to healthy infants.
-
The findings of this study will pave the way for randomised trials of bifidobacterial supplementation in neonates with congenital gastrointestinal surgical conditions.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
McDonald, D. et al. Extreme dysbiosis of the microbiome in critical illness. mSphere 1, e00199–00116 (2016).
Lavallee, C. M. et al. Lipid emulsion formulation of parenteral nutrition affects intestinal microbiota and host responses in neonatal piglets. J. Parenter. Enter. Nutr. 41, 1301–1309 (2017).
Ralls, M. W. et al. Bacterial nutrient foraging in a mouse model of enteral nutrient deprivation: insight into the gut origin of sepsis. Am. J. Physiol. Gastrointest. Liver Physiol. 311, G734–G743 (2016).
Pammi, M. et al. Intestinal dysbiosis in preterm infants preceding necrotizing enterocolitis: a systematic review and meta-analysis. Microbiome 5, 31–45 (2017).
Donnell, S. C. et al. Infection rates in surgical neonates and infants receiving parenteral nutrition: a five-year prospective study. J. Hosp. Infect. 52, 273–280 (2002).
Qin, J. et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 464, 59–65 (2010).
Tan, J. et al. The role of short-chain fatty acids in health and disease. Adv. Immunol. 121, 91–119 (2014).
Dalile, B., Van Oudenhove, L., Vervliet, B. & Verbeke, K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat. Rev. Gastroenterol. Hepatol. 16, 461–478 (2019).
Borewicz, K. et al. Correlating infant faecal microbiota composition and human milk oligosaccharide consumption by microbiota of one-month old breastfed infants. Mol. Nutr. Food Res. 63, e1801214–e1801226 (2019).
Matsuki, T., Watanabe, K. & Tanaka, R. Genus- and species-specific PCR primers for the detection and identification of bifidobacteria. Curr. Issues Intest. Microbiol. 4, 61–69 (2003).
Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 17, 10–12 (2011).
Pruesse, E., Peplies, J. & Glöckner, F. O. SINA: accurate high-throughput multiple sequence alignment of ribosomal RNA genes. Bioinformatics 28, 1823–1829 (2012).
Edgar, R. C. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32, 1792–1797 (2004).
Stamatakis, A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics (Oxf., Engl.) 30, 1312–1313 (2014).
García-Villalba, R. et al. Alternative method for gas chromatography-mass spectrometry analysis of short-chain fatty acids in faecal samples. J. Sep. Sci. 35, 1906–1913 (2012).
Shrivastava, A. & Gupta, V. Methods for the determination of limit of detection and limit of quantitation of the analytical methods. Chron. Young Scientists 2, 21–25 (2011).
Vandenbroucke, J. P. et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Int. J. Surg. 12, 1500–1524 (2014).
Ruiz-Moyano, S. et al. Variation in consumption of human milk oligosaccharides by infant gut-associated strains of Bifidobacterium breve. Appl. Environ. Microbiol. 79, 6040–6049 (2013).
Penders, J. et al. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics 118, 511–521 (2006).
Hidalgo-Cantabrana, C. et al. Bifidobacteria and their health-promoting effects. Microbiol. Spectr. 5, 10–26 (2017).
Milani, C. et al. The first microbial colonizers of the human gut: composition, activities, and health implications of the infant gut microbiota. Microbiol. Mol. Biol. Rev. 81, e00036–00017 (2017).
Chi, C. et al. Longitudinal gut bacterial colonization and its influencing factors of low birth weight infants during the first 3 months of life. Front. Microbiol. 10, 1105–1114 (2019).
Bokulich, N. A. et al. Antibiotics, birth mode, and diet shape microbiome maturation during early life. Sci. Transl. Med. 8, 343ra82 (2016).
Nagpal, R. et al. Ontogenesis of the gut microbiota composition in healthy, full-term, vaginally born and breast-fed infants over the first 3 years of life: a quantitative bird’s-eye view. Front. Microbiol. 8, 1388 (2017).
Fallani, M. et al. Intestinal microbiota of 6-week-old infants across Europe: geographic influence beyond delivery mode, breast-feeding, and antibiotics. J. Pediatr. Gastroenterol. Nutr. 51, 77–84 (2010).
Arrieta, M. C., Stiemsma, L. T., Amenyogbe, N., Brown, E. M. & Finlay, B. The intestinal microbiome in early life: health and disease. Front. Immunol. 5, 427 (2014).
Morrison, D. J. & Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 7, 189–200 (2016).
Macfarlane, G. T. & Macfarlane, S. Bacteria, colonic fermentation, and gastrointestinal health. J. AOAC Int. 95, 50–60 (2012).
Jung, T. H., Park, J. H., Jeon, W. M. & Han, K. S. Butyrate modulates bacterial adherence on LS174T human colorectal cells by stimulating mucin secretion and MAPK signaling pathway. Nutr. Res. Pract. 9, 343–349 (2015).
Levy, M., Thaiss, C. A. & Elinav, E. Metabolites: messengers between the microbiota and the immune system. Genes Dev. 30, 1589–1597 (2016).
De Vuyst, L. & Leroy, F. Cross-feeding between bifidobacteria and butyrate-producing colon bacteria explains bifdobacterial competitiveness, butyrate production, and gas production. Int. J. Food Microbiol. 149, 73–80 (2011).
Rao, S. C. & Patole, S. K. Probiotic research in neonates with congenital gastrointestinal surgical conditions—now is the time. Microb. Biotechnol. 12, 254–258 (2019).
Dermyshi, E. et al. The “Golden Age” of probiotics: a systematic review and meta-analysis of randomized and observational studies in preterm infants. Neonatology 112, 9–23 (2017).
Hagen, P. C. & Skelley, J. W. Efficacy of Bifidobacterium species in prevention of necrotizing enterocolitis in very-low birth weight infants. A systematic review. J. Pediatr. Pharm. Ther. 24, 10–15 (2019).
Powell, W. T. et al. Probiotic administration in infants with gastroschisis: a pilot randomized placebo-controlled trial. J. Pediatr. Gastroenterol. Nutr. 62, 852–857 (2016).
Murakami, H. et al. Intestinal microbiota in neonates requiring urgent surgery: assessing the role of probiotics using fecal DNA sequencing. Pediatr. Surg. Int. 32, 37–43 (2016).
Nakamura, H., Lim, T. & Puri, P. Probiotics for the prevention of Hirschsprung-associated enterocolitis: a systematic review and meta-analysis. Pediatr. Surg. Int. 34, 189–193 (2018).
Okazaki, T. et al. Intestinal microbiota in pediatric surgical cases administered Bifidobacterium breve: a randomized controlled trial. J. Pediatr. Gastroenterol. Nutr. 63, 46–50 (2016).
Lytvyn, L., Quach, K., Banfield, L., Johnston, B. C. & Mertz, D. Probiotics and synbiotics for the prevention of postoperative infections following abdominal surgery: a systematic review and meta-analysis of randomized controlled trials. J. Hosp. Infect. 92, 130–139 (2016).
Yang, Z., Wu, Q., Liu, Y. & Fan, D. Effect of perioperative probiotics and synbiotics on postoperative infections after gastrointestinal surgery: a systematic review with meta-analysis. J. Parenter. Enter. Nutr. 41, 1051–1062 (2017).
Acknowledgements
We sincerely thank the midwives at KEMH for facilitating stool collections of healthy term infants from postnatal wards, nursing staff at PCH for collecting stool samples from surgical infants, Mr. Damber Shrestha for providing data from the neonatal database, Ms. Yen Kok, Ms. Annie Chang, and Ms. Julie Hibbert for processing the stool samples and storing them in deep freezer and Dr. Gayatri Jape and Dr. Abhijeet Rakshasbhuvankar for helping with patient recruitment at KEMH. This study was funded by the PCH Grant and grants from the Centre for Neonatal Research and Education, Neonatal Directorate, KEMH for Women, Western Australia.
Author information
Authors and Affiliations
Contributions
S.C.R.: Conception and design, acquisition of data, analysis and interpretation of data; drafting the article and revising it critically for important intellectual content; and final approval of the version to be published. M.E.: Analysis and interpretation of data; drafting the article and revising it critically for important intellectual content; and final approval of the version to be published. S.K.P.: Conception and design, interpretation of data; revising the article critically for important intellectual content; and final approval of the version to be published. K.N.S.: Conception and design, interpretation of data; revising the draft of the article critically for important intellectual content; and final approval of the version to be published. I.G.: Conception and design, interpretation of data; revising the draft critically for important intellectual content; and final approval of the version to be published. A.K.: Conception and design, interpretation of data; revising the draft critically for important intellectual content; and final approval of the version to be published. B.W.: Analysis and interpretation of data; revising the draft critically for important intellectual content; and final approval of the version to be published. L.C.: Analysis and interpretation of data, revising the draft for important intellectual content; and final approval of the version to be published. P.L.C.: Conception and design, analysis and interpretation of data; revising the draft critically for important intellectual content; and final approval of the version to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Rao, S.C., Esvaran, M., Patole, S.K. et al. Gut microbiota in neonates with congenital gastrointestinal surgical conditions: a prospective study. Pediatr Res 88, 878–886 (2020). https://doi.org/10.1038/s41390-020-0824-7
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-020-0824-7
This article is cited by
-
Nutritional management of neonates who undergo major surgery for gastrointestinal disorders: a joint position paper of the Italian Society of Neonatology (SIN), the Italian Society of Pediatric Surgery (SICP), and the Italian Society of Pediatric Nutrition (SINUPE)
Italian Journal of Pediatrics (2026)
-
Probiotic supplementation for neonates with congenital gastrointestinal surgical conditions: guidelines for future research
Pediatric Research (2023)
-
Probiotic supplementation in neonates with congenital gastrointestinal surgical conditions: a pilot randomised controlled trial
Pediatric Research (2022)