Abstract
Background
Sensitive biomarkers are needed to rapidly identify high-risk infants after hypoxia-ischemia for neuroprotective treatment. Hypotension is a key determinant of hypoxic-ischemic neural injury, and a potent stimulus of humoral pressors including angiotensin-II and arginine vasopressin. We therefore aimed to quantify the relationship between vasopressin and angiotensin-II levels in the latent phase after hypoxia-ischemia induced by umbilical cord occlusion (UCO) with both the severity of preceding hypotension and subsequent neuronal injury.
Methods
Chronically instrumented near-term fetal sheep underwent sham-UCO or UCO for either 15 min or until mean arterial pressure was <8 mmHg. Neuronal injury was assessed after 72 h recovery.
Results
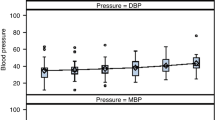
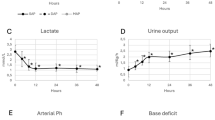
Umbilical cord occlusion was associated with severe hypotension that recovered after UCO; two fetuses developed profound secondary hypotension within 6 h and died. Vasopressin levels but not angiotensin-II were significantly elevated 1–3 h after UCO and were closely associated with the severity of hypotension during UCO and the subsequent severity of neuronal loss in the parasagittal and lateral cortex, caudate nucleus and putamen. The Youden cut-point for vasopressin at 1 h was 180.0 pmol/L, with sensitivity 100% and specificity 92.3% for severe neuronal injury or death.
Conclusion
Vasopressin levels shortly after moderate-severe hypoxia-ischemia may be a useful early biomarker to guide the timely implementation of neuroprotective treatment.
Impact
-
It can be difficuIt to rapidly identify infants who might benefit from therapeutic hypothermia. We investigated whether increases in plasma pressor hormones early after hypoxia-ischemia were biomarkers for neonatal hypoxic-ischemic encephalopathy using near-term fetal sheep.
-
Arginine vasopressin levels were elevated at 1–3 h after hypoxia-ischemia and were predictive of the severity of preceding hypotension and subsequent risk of severe neuronal injury or death after hypoxia-ischemia.
-
Arginine vasopressin may help identify neonates at high risk of hypoxic-ischemic encephalopathy early within the therapeutic window for hypothermia.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Lee, A. C. et al. Intrapartum-related neonatal encephalopathy incidence and impairment at regional and global levels for 2010 with trends from 1990. Pediatr. Res. 74, 50–72 (2013).
Jacobs, S. E. et al. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst. Rev. 1, CD003311 (2013).
Graham, E. M., Everett, A. D., Delpech, J. C. & Northington, F. J. Blood biomarkers for evaluation of perinatal encephalopathy: state of the art. Curr. Opin. Pediatr. 30, 199–203 (2018).
Wassink, G. et al. A working model for hypothermic neuroprotection. J. Physiol. 596, 5641–5654 (2018).
Gunn, A. J., Parer, J. T., Mallard, E. C., Williams, C. E. & Gluckman, P. D. Cerebral histologic and electrocorticographic changes after asphyxia in fetal sheep. Pediatr. Res. 31, 486–491 (1992).
Zubrow, A. B., Daniel, S. S., Stark, R. I., Husain, M. K. & James, L. S. Plasma vasopressin, renin, and catecholamines during nitroprusside-induced maternal and fetal hypotension in sheep. Pediatr. Res. 24, 73–78 (1988).
Giussani, D. A. et al. Effect of carotid denervation on plasma vasopressin levels during acute hypoxia in the late-gestation sheep fetus. J. Physiol. 477, 81–87 (1994).
Giussani, D. A. et al. Adrenergic and vasopressinergic contributions to the cardiovascular response to acute hypoxaemia in the llama fetus. J. Physiol. 515, 233–241 (1999).
Perez, R., Espinoza, M., Riquelme, R., Parer, J. T. & Llanos, A. J. Arginine vasopressin mediates cardiovascular responses to hypoxemia in fetal sheep. Am. J. Physiol. 256, R1011–R1018 (1989).
Lumbers, E. R. et al. Nonimmune hydrops fetalis and activation of the renin-angiotensin system after asphyxia in preterm fetal sheep. Am. J. Physiol. Regul. Integr. Comp. Physiol. 280, R1045–R1051 (2001).
Summanen, M., Back, S., Voipio, J. & Kaila, K. Surge of peripheral Arginine Vasopressin in a rat model of birth asphyxia. Front. Cell. Neurosci. 12, 2 (2018).
Chard, T., Hudson, C. N., Edwards, C. R. & Boyd, N. R. Release of oxytocin and vasopressin by the human foetus during labour. Nature 234, 352–354 (1971).
Summanen, M. et al. Comparison of umbilical serum copeptin relative to erythropoietin and S100B as asphyxia biomarkers at birth. Neonatology 112, 60–66 (2017).
Evers, K. S. & Wellmann, S. Arginine Vasopressin and Copeptin in perinatology. Front. Pediatr. 4, 75 (2016).
Spoljaric, A. et al. Vasopressin excites interneurons to suppress hippocampal network activity across a broad span of brain maturity at birth. Proc. Natl. Acad. Sci. USA 114, E10819–E10828 (2017).
Kasai, M. et al. Early sinusoidal heart rate patterns and heart rate variability to assess hypoxia-ischaemia in near-term fetal sheep. J. Physiol. 597, 5535–5548 (2019).
Barlow, R. M. The foetal sheep: morphogenesis of the nervous system and histochemical aspects of myelination. J. Comp. Neurol. 135, 249–262 (1969).
Drury, P. P. et al. Status epilepticus after prolonged umbilical cord occlusion is associated with greater neural injury in fetal sheep at term-equivalent. PLoS ONE 9, e96530 (2014).
Lear, C. A. et al. Sympathetic neural activation does not mediate heart rate variability during repeated brief umbilical cord occlusions in near-term fetal sheep. J. Physiol. 594, 1265–1277 (2016).
van Bel, F., Roman, C., Klautz, R. J., Teitel, D. F. & Rudolph, A. M. Relationship between brain blood flow and carotid arterial flow in the sheep fetus. Pediatr. Res. 35, 329–333 (1994).
Rademaker, M. T., Charles, C. J., Nicholls, M. G. & Richards, A. M. Interactions of enhanced urocortin 2 and mineralocorticoid receptor antagonism in experimental heart failure. Circ. Heart Fail. 6, 825–832 (2013).
Gluckman, P. D. & Parsons, Y. Stereotaxic method and atlas for the ovine fetal forebrain. J. Dev. Physiol. 5, 101–128 (1983).
Gunn, A. J., Gunn, T. R., de Haan, H. H., Williams, C. E. & Gluckman, P. D. Dramatic neuronal rescue with prolonged selective head cooling after ischemia in fetal lambs. J. Clin. Invest. 99, 248–256 (1997).
Papile, L. A., Rudolph, A. M. & Heymann, M. A. Autoregulation of cerebral blood flow in the preterm fetal lamb. Pediatr. Res. 19, 159–161 (1985).
Green, L. R., McGarrigle, H. H., Bennet, L. & Hanson, M. A. Angiotensin II and cardiovascular chemoreflex responses to acute hypoxia in late gestation fetal sheep. J. Physiol. 507, 857–867 (1998).
Holmes, C. L., Patel, B. M., Russell, J. A. & Walley, K. R. Physiology of vasopressin relevant to management of septic shock. Chest 120, 989–1002 (2001).
Baumann, G. & Dingman, J. F. Distribution, blood transport, and degradation of antidiuretic hormone in man. J. Clin. Invest. 57, 1109–1116 (1976).
Sarkar, S. et al. Relationship between acute kidney injury and brain MRI findings in asphyxiated newborns after therapeutic hypothermia. Pediatr. Res. 75, 431–435 (2014).
Lee, J. K. et al. Relationships between cerebral autoregulation and markers of kidney and liver injury in neonatal encephalopathy and therapeutic hypothermia. J. Perinatol. 37, 938–942 (2017).
Wiriyathian, S., Porter, J. C., Naden, R. P. & Rosenfeld, C. R. Cardiovascular effects and clearance of arginine vasopressin in the fetal lamb. Am. J. Physiol. 245, E24–E31 (1983).
Miao, D. C., Velaphi, S. C., Roy, T., Despain, K. & Rosenfeld, C. R. Metabolism and synthesis of arginine vasopressin in conscious newborn sheep. Am. J. Physiol. Endocrinol. Metab. 295, E672–E677 (2008).
Quaedackers, J. S., Roelfsema, V., Heineman, E., Gunn, A. J. & Bennet, L. The role of the sympathetic nervous system in post-asphyxial intestinal hypoperfusion in the preterm sheep fetus. J. Physiol. 557, 1033–1044 (2004).
Williams, C. E., Gunn, A. J., Mallard, C. & Gluckman, P. D. Outcome after ischemia in the developing sheep brain: an electroencephalographic and histological study. Ann. Neurol. 31, 14–21 (1992).
Hunter, C. J. et al. Key neuroprotective role for endogenous adenosine A1 receptor activation during asphyxia in the fetal sheep. Stroke 34, 2240–2245 (2003).
Jonsson, M., Agren, J., Norden-Lindeberg, S., Ohlin, A. & Hanson, U. Neonatal encephalopathy and the association to asphyxia in labor. Am. J. Obstet. Gynecol. 211, 667 e661–667.e668 (2014).
West, C. R. et al. Antenatal antecedents of moderate or severe neonatal encephalopathy in term infants - a regional review. Aust. N. Z. J. Obstet. Gynaecol. 45, 207–210 (2005).
Westgate, J. A., Gunn, A. J. & Gunn, T. R. Antecedents of neonatal encephalopathy with fetal acidaemia at term. Br. J. Obstet. Gynaecol. 106, 774–782 (1999).
Heida, J. E. et al. Comparison of ex vivo stability of copeptin and vasopressin. Clin. Chem. Lab. Med. 55, 984–992 (2017).
Ruth, V., Fyhrquist, F., Clemons, G. & Raivio, K. O. Cord plasma vasopressin, erythropoietin, and hypoxanthine as indices of asphyxia at birth. Pediatr. Res. 24, 490–494 (1988).
Schlapbach, L. J. et al. Copeptin concentration in cord blood in infants with early-onset sepsis, chorioamnionitis and perinatal asphyxia. BMC Pediatr. 11, 38 (2011).
Kelen, D. et al. Serum copeptin and neuron specific enolase are markers of neonatal distress and long-term neurodevelopmental outcome. PLOS ONE 12, e0184593 (2017).
Acknowledgements
The present study was funded by grants from the Health Research Council of New Zealand (grant number 17/601), the Auckland Medical Research Foundation (grant number 1108004) and New Zealand Lottery Grants Board (grant numbers 209214 and 340855). The funding sources had no role in the design or undertaking of the study, the interpretation of results, writing of the article nor the decision to submit this article for publication.
Author information
Authors and Affiliations
Contributions
These experiments were conducted in the Fetal Physiology and Neuroscience Group laboratory at the University of Auckland. A.J.G., P.P.D. and C.A.L. conceived the hypotheses, experimental design and analysis protocols for the study. C.A.L., P.P.D., J.O.D., and A.J.G. were responsible for data collection. C.A.L., M.K. and P.P.D. performed the analysis. C.A.L. drafted the manuscript. All authors were involved in data interpretation, in the editing and revision of the manuscript, approved the final version of the manuscript and agreed to be accountable for all aspects of the work. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lear, C.A., Kasai, M., Drury, P.P. et al. Plasma vasopressin levels are closely associated with fetal hypotension and neuronal injury after hypoxia-ischemia in near-term fetal sheep. Pediatr Res 88, 857–864 (2020). https://doi.org/10.1038/s41390-020-0845-2
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-020-0845-2