Abstract
Background
Preterm birth and its complications are the primary cause of death among children under the age of 5. Among the survivors, morbidity both perinatally and later in life is common. The dawn of novel technical platforms for comprehensive and sensitive analysis of protein profiles in blood has opened up new possibilities to study both health and disease with significant clinical accuracy, here used to study the preterm infant and the physiological changes of the transition from intrauterine to extrauterine life.
Methods
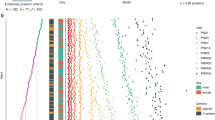
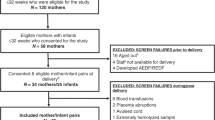
We have performed in-depth analysis of the protein profiles of 14 extremely preterm infants using longitudinal sampling. Medical variables were integrated with extensive profiling of 448 unique protein targets.
Results
The preterm infants have a distinct unified protein profile in blood directly at birth regardless of clinical background; however, the pattern changed profoundly postnatally, expressing more diverse profiles only 1 week later and further on up to term-equivalent age. Clusters of proteins depending on temporal trend were identified.
Conclusion
The protein profiles and the temporal trends here described will contribute to the understanding of the physiological changes in the intrauterine−extrauterine transition, which is essential to adjust early-in-life interventions to prone a normal development in the vulnerable preterm infants.
Impact
-
We have performed longitudinal and in-depth analysis of the protein profiles of 14 extremely preterm infants using a novel multiplex protein analysis platform.
-
The preterm infants had a distinct unified protein profile in blood directly at birth regardless of clinical background.
-
The pattern changed dramatically postnatally, expressing more diverse profiles only 1 week later and further on up to term-equivalent age.
-
Certain clusters of proteins were identified depending on their temporal trend, including several liver and immune proteins.
-
The study contributes to the understanding of the physiological changes in the intrauterine−extrauterine transition.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Data availability
All the data used in the study are available in the supplementary material.
References
Chawanpaiboon, S. et al. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Glob. Health 7, e37–e46 (2019).
Blencowe, H. et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet 379, 2162–2172 (2012).
Romero, R. et al. The preterm parturition syndrome. BJOG 113(Suppl 3), 17–42 (2006).
Goldenberg, R. L. et al. Epidemiology and causes of preterm birth. Lancet 371, 75–84 (2008).
Oza, S. et al. Neonatal cause-of-death estimates for the early and late neonatal periods for 194 countries: 2000−2013. Bull. World Health Organ. 93, 19–28 (2015).
Davey, M. A. et al. Risk-scoring systems for predicting preterm birth with the aim of reducing associated adverse outcomes. Cochrane Database Syst. Rev. p. CD004902 (2015).
You, D. et al. Global, regional, and national levels and trends in under-5 mortality between 1990 and 2015, with scenario-based projections to 2030: a systematic analysis by the UN Inter-agency Group for Child Mortality Estimation. Lancet 386, 2275–2286 (2015).
Olin, A. et al. Stereotypic immune system development in newborn children. Cell 174, 1277–1292.e1214 (2018).
Grisaru-Granovsky, S. et al. Population-based trends in mortality and neonatal morbidities among singleton, very preterm, very low birth weight infants over 16 years. Early Hum. Dev. 90, 821–827 (2014).
Assarsson, E. et al. Homogenous 96-plex PEA immunoassay exhibiting high sensitivity, specificity, and excellent scalability. PLoS ONE 9, e95192 (2014).
Lynch, A. M. et al. The relationship of novel plasma proteins in the early neonatal period with retinopathy of prematurity. Invest. Ophthalmol. Vis. Sci. 57, 5076–5082 (2016).
Suski, M. et al. Prospective plasma proteome changes in preterm infants with different gestational ages. Pediatr. Res. 84, 104–111 (2018).
Najm, S. et al. Effects of a lipid emulsion containing fish oil on polyunsaturated fatty acid profiles, growth and morbidities in extremely premature infants: a randomized controlled trial. Clin. Nutr. ESPEN 20, 17–23 (2017).
Hansen-Pupp, I. et al. Influence of insulin-like growth factor I and nutrition during phases of postnatal growth in very preterm infants. Pediatr. Res. 69, 448–453 (2011).
Kaufman, L. & Rousseeuw, P. J. Finding Groups in Data: An Introduction to Cluster Analysis (John Wiley & Sons, 2009).
Murtagh, F. & Legendre, P. Ward’s hierarchical agglomerative clustering method: which algorithms implement Ward’s criterion? J. Classification 31, 274–295 (2014).
McInnes, L., Healy, J. & Melville, J. Umap: uniform manifold approximation and projection for dimension reduction. Preprint at https://arxiv.org/abs/1802.03426 (2018).
Bates, D. et al. Fitting linear mixed-effects models using lme4. Preprint at https://arxiv.org/abs/1406.5823 (2014).
Yu, G. et al. clusterProfiler: an R package for comparing biological themes among gene clusters. Omics: J. Integr. Biol. 16, 284–287 (2012).
Ritchie, M. E. et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 43, e47–e47 (2015).
Uhlen, M. et al. A genome-wide transcriptomic analysis of protein-coding genes in human blood cells. Science 366.6472 (2019).
Uhlén, M. et al. Tissue-based map of the human proteome. Science 347.6220, 1260419 (2015).
Luo, W. et al. GAGE: generally applicable gene set enrichment for pathway analysis. BMC Bioinform. 10, 161 (2009).
Hannan, J. P. The structure-function relationships of complement receptor type 2 (CR2; CD21). Curr. Protein Pept. Sci. 17, 463–487 (2016).
Ma, Y. J. et al. Soluble collectin-12 (CL-12) is a pattern recognition molecule initiating complement activation via the alternative pathway. J. Immunol. 195, 3365–3373 (2015).
Guasti, L. et al. Elevated FGF21 leads to attenuated postnatal linear growth in preterm infants through GH resistance in chondrocytes. J. Clin. Endocrinol. Metab. 99, E2198–E2206 (2014).
Acencio, M. L., Lægreid, A. & Kuiper, M. et al. The Gene Ontology Resource: 20 years and still GOing strong. Nucleic Acids Res. 47, D330−D338 (2019).
Menzies-Gow, A. et al. Eotaxin (CCL11) and eotaxin-2 (CCL24) induce recruitment of eosinophils, basophils, neutrophils, and macrophages as well as features of early- and late-phase allergic reactions following cutaneous injection in human atopic and nonatopic volunteers. J. Immunol. 169, 2712–2718 (2002).
Huang da, W., Sherman, B. T. & Lempicki, R. A. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 37, 1–13 (2009).
Wang, X. M. et al. Fibroblast activation protein increases apoptosis, cell adhesion, and migration by the LX-2 human stellate cell line. Hepatology 42, 935–945 (2005).
Wisgrill, L. et al. Endothelial cells of extremely premature infants display impaired immune response after proinflammatory stimulation. Pediatr. Res. 83, 128–134 (2018).
Yakovleva, A. A. et al. Dipeptidylpeptidase 4 (DPP4, CD26) activity in the blood serum of term and preterm neonates with cerebral ischemia. Neuropeptides 52, 113–117 (2015).
Walker, J. C. et al. Development of lymphocyte subpopulations in preterm infants. Scand. J. Immunol. 73, 53–58 (2011).
Soerensen, C. M. et al. Purification, characterization and immunolocalization of porcine surfactant protein D. Immunology 114, 72–82 (2005).
Traustadottir, G. A. et al. The imprinted gene Delta like non-canonical Notch ligand 1 (Dlk1) is conserved in mammals, and serves a growth modulatory role during tissue development and regeneration through Notch dependent and independent mechanisms. Cytokine Growth Factor Rev. 46, 17–27 (2019).
Tezze, C., Romanello, V. & Sandri, M. FGF21 as modulator of metabolism in health and disease. Front. Physiol. 10, 419 (2019).
Tanaka, T., Narazaki, M. & Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 6, a016295 (2014).
Acknowledgements
The main funding was provided from the Erling Persson Foundation (KCAP). We would like to thank the Plasma Profiling Facility at SciLifeLab in Stockholm for conducting the Olink analyses. We acknowledge the entire staff of the Human Protein Atlas program and the Science for Life Laboratory for their valuable contributions.
Author information
Authors and Affiliations
Contributions
M.U., L.F. and A.H. conceived and designed the analysis. A.E., A.H., G.H., H.D. and M.J.K. collected and contributed data to the study. W.Z., H.D., A.T., L.F., M.J.K. and M.U. performed the data analysis. A.H., G.H. and A.E. supplied clinical material. M.U., W.Z., H.D. and L.F. drafted the manuscript. All authors discussed the analyses, results and contributed to the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics statement
The trial was approved by the Regional Ethical Board, Gothenburg (Dnr 303-11, T336-18) at the University of Gothenburg. Informed written consent was obtained for all participants from their parents or guardians.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Zhong, W., Danielsson, H., Tebani, A. et al. Dramatic changes in blood protein levels during the first week of life in extremely preterm infants. Pediatr Res 89, 604–612 (2021). https://doi.org/10.1038/s41390-020-0912-8
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41390-020-0912-8
This article is cited by
-
Plasma amino acids after human milk fortification and associations with growth in preterm infants
Pediatric Research (2025)
-
Neurofilament light chain associates with IVH and ROP in extremely preterm infants
Pediatric Research (2025)
-
Antisecretory factor in breastmilk is associated with reduced incidence of sepsis in preterm infants
Pediatric Research (2024)
-
The development of blood protein profiles in extremely preterm infants follows a stereotypic evolution pattern
Communications Medicine (2023)
-
Young infants display heterogeneous serological responses and extensive but reversible transcriptional changes following initial immunizations
Nature Communications (2023)