Abstract
Background
In premature infants, we investigated whether the duration of extrauterine development influenced autonomic nervous system (ANS) maturation.
Methods
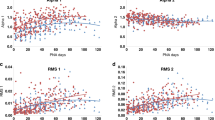
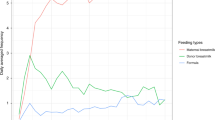
We performed a longitudinal cohort study of ANS maturation in preterm infants. Eligibility included birth gestational age (GA) < 37 weeks, NICU admission, and expected survival. The cohort was divided into three birth GA groups: Group 1 (≤29 weeks), Group 2 (30–33 weeks), and Group 3 (≥34 weeks). ECG data were recorded weekly and analyzed for sympathetic and parasympathetic tone using heart rate variability (HRV). Quantile regression modeled the slope of ANS maturation among the groups by postnatal age to term-equivalent age (TEA) (≥37 weeks).
Results
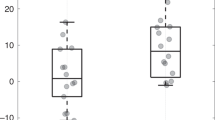
One hundred infants, median (Q1−Q3) birth GA of 31.9 (28.7–33.9) weeks, were enrolled: Group 1 (n = 35); Group 2 (n = 40); and Group 3 (n = 25). Earlier birth GA was associated with lower sympathetic and parasympathetic tone. However, the rate of autonomic maturation was similar, and at TEA there was no difference in HRV metrics across the three groups. The majority of infants (91%) did not experience significant neonatal morbidities.
Conclusion
Premature infants with low prematurity-related systemic morbidity have maturational trajectories of ANS development that are comparable across a wide range of ex-utero durations regardless of birth GA.
Impact
-
Heart rate variability can evaluate the maturation of the autonomic nervous system.
-
Metrics of both the sympathetic and parasympathetic nervous system show maturation in the premature extrauterine milieu.
-
The autonomic nervous system in preterm infants shows comparable maturation across a wide range of birth gestational ages.
-
Preterm newborns with low medical morbidity have maturation of their autonomic nervous system while in the NICU.
-
Modern NICU advances appear to support autonomic development in the preterm infant.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Mulkey, S. B. & Plessis, A. D. The critical role of the central autonomic nervous system in fetal-neonatal transition. Semin. Pediatr. Neurol. 28, 29–37 (2018).
Longin, E., Gerstner, T., Schaible, T., Lenz, T. & Konig, S. Maturation of the autonomic nervous system: differences in heart rate variability in premature vs. term infants. J. Perinat. Med. 34, 303–308 (2006).
Fyfe, K. L. et al. The effect of gestational age at birth on post-term maturation of heart rate variability. Sleep 38, 1635–1644 (2015).
Task Force of the European Society of Cardiology the North American Society of Pacing Electrophysiology. Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Eur. Heart J. 93, 1043–1065 (1996).
Malliani, A., Lombardi, F. & Pagani, M. Power spectrum analysis of heart rate variability: a tool to explore neural regulatory mechanisms. Br. Heart J. 71, 1–2 (1994).
Mulkey, S. B. et al. Autonomic nervous system depression at term in neurologically normal premature infants. Early Hum. Dev. 123, 11–16 (2018).
Yiallourou, S. R., Witcombe, N. B., Sands, S. A., Walker, A. M. & Horne, R. S. The development of autonomic cardiovascular control is altered by preterm birth. Early Hum. Dev. 89, 145–152 (2013).
Tuzcu, V., Nas, S., Ulusar, U., Ugur, A. & Kaiser, J. R. Altered heart rhythm dynamics in very low birth weight infants with impending intraventricular hemorrhage. Pediatrics 123, 810–815 (2009).
Massaro, A. N. et al. Heart rate variability in encephalopathic newborns during and after therapeutic hypothermia. J. Perinatol. 34, 836–841 (2014).
Fairchild, K. D. & O’Shea, T. M. Heart rate characteristics: physiomarkers for detection of late-onset neonatal sepsis. Clin. Perinatol. 37, 581–598 (2010).
Al-Shargabi, T. et al. Changes in autonomic tone in premature infants developing necrotizing enterocolitis. Am. J. Perinatol. 35, 1079–1086 (2018).
Nino, G. et al. Premature infants rehospitalized because of an apparent life-threatening event had distinctive autonomic developmental trajectories. Am. J. Respir. Crit. Care Med. 194, 379–381 (2016).
Mulkey, S. B. & du Plessis, A. J. Autonomic nervous system development and its impact on neuropsychiatric outcome. Pediatr. Res. 85, 120–126 (2019).
Haraldsdottir, K. et al. Heart rate recovery after maximal exercise is impaired in healthy young adults born preterm. Eur. J. Appl. Physiol. 119, 857–866 (2019).
Patural, H. et al. Autonomic cardiac control of very preterm newborns: a prolonged dysfunction. Early Hum. Dev. 84, 681–687 (2008).
Thiriez, G. et al. Altered autonomic control in preterm newborns with impaired neurological outcomes. Clin. Auton. Res. 25, 233–242 (2015).
de Bijl-Marcus, K., Brouwer, A. J., De Vries, L. S., Groenendaal, F. & Wezel-Meijler, G. V. Neonatal care bundles are associated with a reduction in the incidence of intraventricular haemorrhage in preterm infants: a multicentre cohort study. Arch. Dis. Child Fetal Neonatal Ed. (2019). https://doi.org/10.1136/archdischild-2018-316692. [Epub ahead of print]
Ulusar, U. D. et al. Adaptive rule based fetal QRS complex detection using Hilbert transform. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2009, 4666–4669 (2009).
Fyfe, K. L., Yiallourou, S. R., Wong, F. Y. & Horne, R. S. The development of cardiovascular and cerebral vascular control in preterm infants. Sleep. Med. Rev. 18, 299–310 (2014).
Peng, C. K., Havlin, S., Stanley, H. E. & Goldberger, A. L. Quantification of scaling exponents and crossover phenomena in nonstationary heartbeat time series. Chaos 5, 82–87 (1995).
Govindan, R. B., Massaro, A. N., Niforatos, N. & du Plessis, A. Mitigating the effect of non-stationarity in spectral analysis-an application to neonate heart rate analysis. Comput. Biol. Med. 43, 2001–2006 (2013).
Govindan, R. B., Preissl, H., Eswaran, H., Campbell, J. Q. & Lowery, C. L. Detrended fluctuation analysis of short data sets: an application to fetal cardiac. Phys. D: Nonlinear Phenom. 226, 23–31 (2007).
Patural, H. et al. Birth prematurity determines prolonged autonomic nervous system immaturity. Clin. Auton. Res. 14, 391–395 (2004).
Clairambault, J., Curzi-Dascalova, L., Kauffmann, F., Medigue, C. & Leffler, C. Heart rate variability in normal sleeping full-term and preterm neonates. Early Hum. Dev. 28, 169–183 (1992).
Tulppo, M. P. et al. Physiological background of the loss of fractal heart rate dynamics. Circulation 112, 314–319 (2005).
Bohanon, F. J. et al. Heart rate variability analysis is more sensitive at identifying neonatal sepsis than conventional vital signs. Am. J. Surg. 210, 661–667 (2015).
Goudjil, S. et al. Patent ductus arteriosus in preterm infants is associated with cardiac autonomic alteration and predominant parasympathetic stimulation. Early Hum. Dev. 89, 631–634 (2013).
Sanders, M. R. & Hall, S. L. Trauma-informed care in the newborn intensive care unit: promoting safety, security and connectedness. J. Perinatol. 38, 3–10 (2018).
Gomes, E. et al. Autonomic responses of premature newborns to body position and environmental noise in the neonatal intensive care unit. Rev. Bras. Ter. Intensiv. 31, 296–302 (2019).
Feldman, R., Rosenthal, Z. & Eidelman, A. I. Maternal-preterm skin-to-skin contact enhances child physiologic organization and cognitive control across the first 10 years of life. Biol. Psychiatry 75, 56–64 (2014).
Tarvainen, M. P., Niskanen, J. P., Lipponen, J. A., Ranta-Aho, P. O. & Karjalainen, P. A. Kubios HRV-heart rate variability analysis software. Comput. Methods Prog. Biomed. 113, 210–220 (2014).
Shayani, L. A., da Cruz, C. J., Porto, L. G. G. & Molina, G. E. Cardiac autonomic function in the first hours of postnatal life: an observational cross-sectional study in term neonates. Pediatr. Cardiol. 40, 1703–1708 (2019).
Mulkey, S. B. et al. The effect of labor and delivery mode on electrocortical and brainstem autonomic function during neonatal transition. Sci. Rep. 9, 11020 (2019).
Aye, C. Y. L. et al. Neonatal autonomic function after pregnancy complications and early cardiovascular development. Pediatr. Res. 84, 85–91 (2018).
Acknowledgements
We greatly appreciate the support of Inova Women and Children’s Hospital, Fairfax, VA and the families who allowed us to study their newborn infants. This study was supported by the Children’s National Inova Collaborative (CNICA) Research Program, through institutional support from Children’s National Hospital, Washington, DC and the Inova Health System, Fairfax, VA. S.B.M. received support by Award Numbers UL1TR001876 and KL2TR001877 from the NIH National Center for Advancing Translational Sciences. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
S.B.M.: designed the study, obtained funding, acquired and analyzed the data, drafted the manuscript, critically revised the manuscript, approved the final version for publication. R.B.G.: helped designed the study, analyzed the data, critically revised the manuscript, approved the final version for publication. L.H.: helped design the study, acquired data, critically revised the manuscript, approved the final version for publication. T.A.-S.: helped design the study, acquired data, analyzed data, critically revised the manuscript, approved the final version for publication. N.H., M.B.J., R.M.: biostatistical study design, analyzed data, critically revised the manuscript, approved the final version for publication. C.B.S.: helped design the study, acquired data, critically revised the manuscript, approved the final version for publication. A.E., S.R., S.D.S.: acquired data, critically revised the manuscript, approved the final version for publication. A.K.: contributed to participant enrollment, acquired data, critically revised the manuscript, approved the final version for publication. G.L.M., A.J.d.P.: helped develop the concept of the study, critically revised the manuscript, approved the final version for publication. R.B.: helped develop the concept of the study, contributed to participant enrollment, critically revised the manuscript, approved the final version for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Informed consent
Patient written informed consent was obtained for the study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mulkey, S.B., Govindan, R.B., Hitchings, L. et al. Autonomic nervous system maturation in the premature extrauterine milieu. Pediatr Res 89, 863–868 (2021). https://doi.org/10.1038/s41390-020-0952-0
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41390-020-0952-0
This article is cited by
-
Autonomic development in preterm infants is associated with morbidity of prematurity
Pediatric Research (2022)
-
In infants with congenital heart disease autonomic dysfunction is associated with pre-operative brain injury
Pediatric Research (2022)
-
Vital signs as physiomarkers of neonatal sepsis
Pediatric Research (2022)