Abstract
Background
Dobutamine is particularly suited to treatment of haemodynamic insufficiency caused by increased peripheral vascular resistance and myocardial dysfunction in the preterm infant. Knowledge of the elimination half-life is essential to estimate the steady state when its efficacy/safety can be evaluated.
Methods
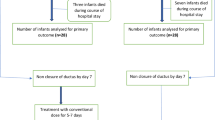
Analysis of pharmacokinetic data in ten preterm newborns treated with a new neonatal formulation of dobutamine (IMP) after screening for haemodynamic insufficiency within the first 72 h from birth. Blood samples were withdrawn at the end of IMP infusion and at a random time after the end of infusion (5 min, 15 min, 45 min, 2 h and 6 h). IMP concentration in each sample was measured by ultra-high performance liquid chromatography with electrochemical detection.
Results
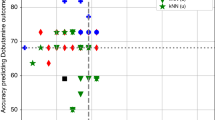
Median duration of IMP infusion was 37.7 h (IQR 21.2). Calculated IMP half-life ranged between 3.06 and 36.1 min (median 10.6 min), leading to a time to reach the steady-state concentration between 15 min and >2 h. Adverse events were not related to IMP.
Conclusions
The wide variability in dobutamine metabolism in preterm infants requires awareness about the risk of under- or overtreatment. A delay of up to 3 h might be required before drawing blood samples to evaluate the effective dose.
Impact
-
Small trials suggest dobutamine as the optimal drug in the preterm infant with haemodynamic insufficiency after birth.
-
Age-related differences in drug pharmacokinetics may result in suboptimal treatments.
-
The lack of formal studies in preterms results in inadequate data on efficacy and safety.
-
This study provides data on the variability of the elimination half-life of dobutamine in the very preterm infant during transitional circulation.
-
There is a wide variation in the time to reach the plasma concentration corresponding to steady state, the moment when its efficacy/safety can be reliably evaluated.
-
This information is crucial for planning future trials on cardiovascular support.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Ruggieri, L. et al. Successful private-public funding of paediatric medicines research: lessons from the EU programme to fund research into off-patent medicines. Eur. J. Pediatr. 174, 481–491 (2015).
Batton, B. et al. Blood pressure, anti-hypotensive therapy, and neurodevelopment in extremely preterm infants. J. Pediatr. 154, 351–357 (2009).
Faust, K. et al. Short-term outcome of very-low birth weight infants with arterial hypotension in the first 24 h of life. Arch. Dis. Child Fetal Neonatal Ed. 100, F388–F392 (2015).
Kluckow, M. & Evans, N. Low superior vena cava flow and intraventricular haemorrhage in preterm infants. Arch. Dis. Child Fetal Neonatal Ed. 82, F188–F194 (2000).
Kluckow, M. Low systemic blood flow and pathophysiology of the preterm transitional circulation. Early Hum. Dev. 81, 429–437 (2005).
Osborn, D. A. et al. Low superior vena cava flow and effect of inotropes on neurodevelopment to 3 years in preterm infants. Pediatrics 120, 372–380 (2007).
Kluckow, M. & Seri, I. in Hemodynamics and Cardiology. Neonatology Questions and Controversies, 2nd edn. (eds Kleinman, C. S. & Seri, I.) 237−267 (Elsevier Saunders, Philadelphia, 2012).
Osborn, D., Evans, N. & Kluckow, M. Randomized trial of dobutamine versus dopamine in preterm infants with low systemic blood flow. J. Pediatr. 140, 183–191 (2002).
Bravo, M. C. et al. Randomized, placebo-controlled trial of dobutamine for low superior vena cava flow in infants. J. Pediatr. 167, 572–578 (2015).
Roze, J. C. et al. Response to dobutamine and dopamine in the hypotensive very preterm infant. Arch. Dis. Child 69(1 Spec No), 59–63 (1993).
Hentschel, R. et al. Impact on blood pressure and intestinal perfusion of dobutamine or dopamine in hypotensive preterm infants. Biol. Neonate 68, 318–324 (1995).
Ruffolo, R. R. Jr. The pharmacology of dobutamine. Am. J. Med. Sci. 294, 244–248 (1987).
European Medicines Agency. Committee for medicinal products for human use. Reflection paper: formulations of choice for the paediatric population. EMEA/CHMP/PEG/194810/2005.
European Medicines Agency. Opinion of the Paediatric Committee on the acceptance of a modification of an agreed Paediatric Investigation Plan. EMEA-001262-PIP01-12-M02. London, 14 October 2016.
Rowland M., Toze T. N. Clinical Pharmacokinetics: Concepts and Applications, 3rd edn, 83−105 (Williams and Wilkins, Philadelphia, 1995).
European Medicines Agency. Guideline on bioanalytical method validation. EMEA/CHMP/EWP/192217/2009. Rev.1 Corr.2.
European Medicines Agency. Guideline on Pharmacokinetic studies in man. https://www.ema.europa.eu/en/documents/scientific-guideline/pharmacokinetic-studies-man_en.pdf.
Devries, L. S., Eken, P. & Dubowitz, L. M. S. The spectrum of leukomalacia using cranial ultrasound. Behav. Brain Res. 49, 1–6 (1992).
Levene, M. I. Measurement of the growth of the lateral ventricles in preterm infants with real-time ultrasound. Arch. Dis. Child. 56, 900–904 (1981).
Pellicer, A. et al. Cardiovascular support for low birth weight infants and cerebral hemodynamics: a randomized, blinded, clinical trial. Pediatrics 115, 1501–1512 (2005).
Myohanen, T. T. & Mannisto, P. T. Distribution and functions of catechol-O-methyltransferase proteins: do recent findings change the picture? Int. Rev. Neurobiol. 95, 29–47 (2010).
Martinez, A. M., Padbury, J. F. & Thio, S. Dobutamine pharmacokinetics and cardiovascular responses in critically ill neonates. Pediatrics 89, 47–51 (1992).
Banner, W. Jr, Vernon, D. D., Minton, S. D. & Dean, J. M. Nonlinear dobutamine pharmacokinetics in a pediatric population. Crit. Care Med. 19, 871–873 (1991).
Schwartz, P. H., Eldadah, M. K. & Newth, C. J. The pharmacokinetics of dobutamine in pediatric intensice care unit patients. Drug Metab. Dispos. 19, 614–619 (1991).
Habib, D. M. et al. Dobutamine pharmacokinetics and pharmacodynamics in pediatric intensive care patients. Crit. Care Med. 20, 601–608 (1992).
Berg, R. A. et al. Dobutamine pharmacokinetics and pharmacodynamics in normal children and adolescents. J. Pharm. Exp. Ther. 265, 1232–1238 (1993).
Berg, R. A., Donnerstein, R. L. & Padbury, J. F. Dobutamine infusions in stable, critically ill children: pharmacokinetics and hemodynamic actions. Crit. Care Med. 21, 678–686 (1993).
Berg, R. A. & Padbury, J. F. Sulfoconjugation and renal excretion contribute to the interpatient variation of exogenous catecholamine clearance in critically ill children. Crit. Care Med. 25, 1247–1251 (1997).
Hallik, M. et al. Population pharmacokinetics and pharmacodynamics of dobutamine in neonates on the first days of life. Br. J. Clin. Pharm. 86, 318–328 (2020).
Mielgo, V. E. et al. Hemodynamic and metabolic effects of a new pediatric dobutamine formulation in hypoxic newborn pigs. Pediatr. Res. 81, 511–518 (2017).
Richardson, D. K., Corcoran, J. D., Escobar, G. J. & Lee, S. K., for The Canadian NICU Network, The Kaiser Permanente Neonatal Minimum Data Set Wide Area Network, and The SNAP-II Study Group. SNAP-II and SNAPPE-II: simplified newborn illness severity and mortality risk scores. J. Pediatr. 138, 92–100 (2001).
Acknowledgements
The authors thank all participants, parents, and healthcare providers. This work was supported by the European Union Frame Programme Seventh grant number FP7: HEALTH-2011.4.2-1 [Investigator-driven clinical trials on off-patent medicines for children].
Author information
Authors and Affiliations
Consortia
Contributions
A.P., V.J., F.C., and H.R. conceptualised and designed the study; C.G., H.R.-A., A.K. and A.S. supported the study design; A.P., R.F., M.C.B., P.L.O., L.S., and M.Y. assisted with acquisition of the data; A.K. and A.S. analysed the data; A.P. wrote the initial draft; A.P., V.J., C.G., H.R.-A., A.K., A.S. and H.R. provided administrative, technical, and material support. All authors critically reviewed the revised versions of the paper and approved the final draft for submission. All authors had access to the data and contributed to the submitted report. A.P. is the guarantor.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
Ethics approval for this study was obtained from the Ethics Committee for Clinical Research at La Paz University Hospital (HULP 3969) and the NRES Committee South East Coast-Brighton and Sussex (13/LO/1426).
Patient consent
Patient consent was obtained for this study.
Transparency
A.P. affirms that the manuscript is honest, accurate, and transparent account of the study being reported, that no important aspects have been omitted, and that any discrepancies from the study as planned have been explained.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pellicer, A., Fernández, R., Jullien, V. et al. Pharmacokinetic study (phase I−II) of a new dobutamine formulation in preterm infants immediately after birth. Pediatr Res 89, 981–986 (2021). https://doi.org/10.1038/s41390-020-1009-0
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-020-1009-0