Abstract
Background
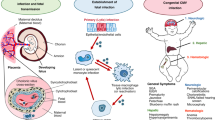
Cytomegalovirus (CMV) is a leading infectious cause of neurologic deficits, both in the settings of congenital and perinatal infection, but few animal models exist to study neurodevelopmental outcomes. This study examined the impact of neonatal guinea pig CMV (GPCMV) infection on spatial learning and memory in a Morris water maze (MWM) model.
Methods
Newborn pups were challenged intraperitoneally (i.p.) with a pathogenic red fluorescent protein-tagged GPCMV, or sham inoculated. On days 15–19 post infection (p.i.), pups were tested in the MWM. Viral loads were measured in blood and tissue by quantitative PCR (qPCR), and brain samples collected at necropsy were examined by histology and immunohistochemistry.
Results
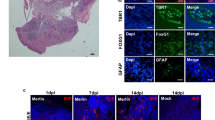
Viremia (DNAemia) was detected at day 3 p.i. in 7/8 challenged animals. End-organ dissemination was observed, by qPCR, in the lung, liver, and spleen. CD4-positive (CD4+) and CD8-positive (CD8+) T cell infiltrates were present in brains of challenged animals, particularly in periventricular and hippocampal regions. Reactive gliosis and microglial nodules were observed. Statistically significant spatial learning and memory deficits were observed by MWM, particularly for total maze distance traveled (p < 0.0001).
Conclusion
Neonatal GPCMV infection in guinea pigs results in cognitive defects demonstrable by the MWM. This neonatal guinea pig challenge model can be exploited for studying antiviral interventions.
Impact
-
CMV impairs neonatal neurocognition and memory in the setting of postnatal infection.
-
The MWM can be used to examine memory and learning in a guinea pig model of neonatal CMV infection.
-
CD4+ and CD8+ T cells infiltrate the brain following neonatal CMV challenge.
-
This article demonstrates that the MWM can be used to evaluate memory and learning after neonatal GPCMV challenge.
-
The guinea pig can be used to examine central nervous system pathology caused by neonatal CMV infection and this attribute may facilitate the study of vaccines and antivirals.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Cheeran, M. C., Lokensgard, J. R. & Schleiss, M. R. Neuropathogenesis of congenital cytomegalovirus infection: disease mechanisms and prospects for intervention. Clin. Microbiol. Rev. 22, 99–126 (2009).
Teissier, N. et al. Cytomegalovirus-induced brain malformations in fetuses. J. Neuropathol. Exp. Neurol. 73, 143–158 (2014).
Gabrielli, L. et al. Congenital cytomegalovirus infection: patterns of fetal brain damage. Clin. Microbiol. Infect. 18, E419–E427 (2012).
Lanzieri, T. M. et al. Long-term outcomes of children with symptomatic congenital cytomegalovirus disease. J. Perinatol. 37, 875–80. (2017).
Dollard, S. C., Grosse, S. D. & Ross, D. S. New estimates of the prevalence of neurological and sensory sequelae and mortality associated with congenital cytomegalovirus infection. Rev. Med. Virol. 17, 355–363 (2007).
Weimer, K. E. D., Kelly, M. S., Permar, S. R., Clark, R. H. & Greenberg R. G. Association of adverse hearing, growth, and discharge age outcomes with postnatal cytomegalovirus infection in infants with very low birth weight. JAMA Pediatr. https://doi.org/10.1001/jamapediatrics.2019.4532 (2019).
Schleiss, M. R. Breast milk-acquired cytomegalovirus in premature infants: uncertain consequences and unsolved biological questions. JAMA Pediatr. https://doi.org/10.1001/jamapediatrics.2019.4538 (2019).
Schleiss, M. R. Nonprimate models of congenital cytomegalovirus (CMV) infection: gaining insight into pathogenesis and prevention of disease in newborns. ILAR J. 47, 65–72 (2006).
Bravo, F. J., Bourne, N., Schleiss, M. R. & Bernstein, D. I. An animal model of neonatal cytomegalovirus infection. Antivir. Res. 60, 41–49 (2003).
Tsutsui, Y., Kosugi, I. & Kawasaki, H. Neuropathogenesis in cytomegalovirus infection: indication of the mechanisms using mouse models. Rev. Med. Virol. 15, 327–345 (2005).
Slavuljica, I. et al. Immunobiology of congenital cytomegalovirus infection of the central nervous system—the murine cytomegalovirus model. Cell Mol. Immunol. 12, 180–191 (2015).
Koontz, T. et al. Altered development of the brain after focal herpesvirus infection of the central nervous system. J. Exp. Med. 205, 423–435 (2008).
Brizić, I. et al. CD4 T cells are required for maintenance of CD8 TRM cells and virus control in the brain of MCMV-infected newborn mice. Med. Microbiol. Immunol. 208, 487–494 (2019).
Sung, C. Y. W. et al. Virus-induced cochlear inflammation in newborn mice alters auditory function. JCI Insight 4, e128878 (2019).
Ikuta, K. et al. Restricted infection of murine cytomegalovirus (MCMV) in neonatal mice with MCMV-induced sensorineural hearing loss. J. Clin. Virol. 69, 138–145 (2015).
Morris, R. G. M. Spatial localization does not require the presence of local cues. Learn. Motiv. 12, 239–260 (1981).
D’Hooge, R. & De Deyn, P. P. Applications of the Morris water maze in the study of learning and memory. Brain Res. Brain Res. Rev. 36, 60–90 (2001).
Armien, A. G. et al. Chronic cortical and subcortical pathology with associated neurological deficits ensuing experimental herpes encephalitis. Brain Pathol. 20, 738–750 (2010).
Buenz, E. J., Rodriguez, M. & Howe, C. L. Disrupted spatial memory is a consequence of picornavirus infection. Neurobiol. Dis. 24, 266–273 (2006).
Byrnes, M. L., Richardson, D. P., Brien, J. F., Reynolds, J. N. & Dringenberg, H. C. Spatial acquisition in the Morris water maze and hippocampal long-term potentiation in the adult guinea pig following brain growth spurt–prenatal ethanol exposure. Neurotoxicol. Teratol. 26, 543–551 (2004).
McAdam, T. D., Brien, J. F., Reynolds, J. N. & Dringenberg, H. C. Altered water-maze search behavior in adult guinea pigs following chronic prenatal ethanol exposure: lack of mitigation by postnatal fluoxetine treatment. Behav. Brain Res. 191, 202–209 (2008).
Fiset, C. R. F., Surette, M. E. & Fiset, S. Prenatal iron deficiency in guinea pigs increases locomotor activity but does not influence learning and memory. PLoS ONE 10, e0133168 (2015).
LeBlanc, C. P., Fiset, S., Surette, M. E., Turgeon O’Brien, H. & Rioux, F. M. Maternal iron deficiency alters essential fatty acid and eicosanoid metabolism and increases locomotion in adult guinea pig offspring. J. Nutr. 139, 1653–1659 (2009).
Mamczarz, J. et al. Spatial learning impairment in prepubertal guinea pigs prenatally exposed to the organophosphorus pesticide chlorpyrifos: toxicological implications. Neurotoxicology 56, 17–28 (2016).
McVoy, M. A. et al. Repair of a mutation disrupting the guinea pig cytomegalovirus pentameric complex acquired during fibroblast passage restores pathogenesis in immune-suppressed guinea pigs and in the context of congenital infection. J. Virol. 90, 7715–7727 (2016).
Bierle, C. J., Anderholm, K. M., Wang, J. B., McVoy, M. A. & Schleiss, M. R. Targeted mutagenesis of guinea pig cytomegalovirus using CRISPR/Cas9-mediated gene editing. J. Virol. 90, 6989–6998 (2016).
Booss, J., Winkler, S. R., Griffith, B. P. & Kim, J. H. Viremia and glial nodule encephalitis after experimental systemic cytomegalovirus infection. Lab Invest. 61, 644–649 (1989).
Swanson, E. C. et al. Comparison of monovalent glycoprotein B with bivalent gB/pp65 (GP83) vaccine for congenital cytomegalovirus infection in a guinea pig model: Inclusion of GP83 reduces gB antibody response but both vaccine approaches provide equivalent protection against pup mortality. Vaccine 33, 4013–4018 (2015).
Espinoza, J. A. et al. Impaired learning resulting from respiratory syncytial virus infection. Proc. Natl Acad. Sci. USA 110, 9112–9117 (2013).
Jurgens, H. A., Amancherla, K. & Johnson, R. W. Influenza infection induces neuroinflammation, alters hippocampal neuron morphology, and impairs cognition in adult mice. J. Neurosci. 32, 3958–3968 (2012).
Chen, J. et al. Long-term impact of intrauterine MCMV infection on development of offspring nervous system. J. Huazhong Univ. Sci. Technol. Med. Sci. 31, 371–375 (2011).
Lokensgard, J. R. et al. Chronic reactive gliosis following regulatory T cell depletion during acute MCMV encephalitis. Glia 63, 1982–1996 (2015).
Dringenberg, H. C., Richardson, D. P., Brien, J. F. & Reynolds, J. N. Spatial learning in the guinea pig: cued versus non-cued learning, sex differences, and comparison with rats. Behav. Brain Res. 124, 97–101 (2001).
Booss, J., Dann, P. R., Griffith, B. P. & Kim, J. H. Glial nodule encephalitis in the guinea pig: serial observations following cytomegalovirus infection. Acta Neuropathol. 75, 465–473 (1988).
Griffith, B. P., Lucia, H. L. & Hsiung, G. D. Brain and visceral involvement during congenital cytomegalovirus infection of guinea pigs. Pediatr. Res. 16, 455–459 (1982).
Bantug, G. R. et al. CD8+ T lymphocytes control murine cytomegalovirus replication in the central nervous system of newborn animals. J. Immunol. 181, 2111–2123 (2008).
Cekinović, D. et al. Passive immunization reduces murine cytomegalovirus-induced brain pathology in newborn mice. J. Virol. 82, 12172–12180 (2008).
Mutnal, M. B., Hu, S. & Lokensgard, J. R. Persistent humoral immune responses in the CNS limit recovery of reactivated murine cytomegalovirus. PLoS ONE 7, e33143 (2012).
Acknowledgements
We would like to thank Dr. Thomas Pengo, for his assistance with the use of the Imaris software and Steve Ricchio, who helped us to obtain the confocal images. This work was supported by NIH HD079918, HD098866, and NS038836.
Author information
Authors and Affiliations
Contributions
Substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data: C.F.-A., L.E.M., M.A.M., J.R.L., S.H., M.A.B., K.M.A., B.C.J., and M.R.S. Drafting the article or revising it critically for important intellectual content: C.F.-A., L.E.M., M.A.M., J.R.L., S.H., M.A.B., K.M.A., and M.R.S. Final approval of the version to be published: C.F.-A., L.E.M., M.A.M., J.R.L., S.H., M.A.B., K.M.A., B.C.J., and M.R.S.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fernández-Alarcón, C., Meyer, L.E., McVoy, M.A. et al. Impairment in neurocognitive function following experimental neonatal guinea pig cytomegalovirus infection. Pediatr Res 89, 838–845 (2021). https://doi.org/10.1038/s41390-020-1010-7
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-020-1010-7
This article is cited by
-
Neuron-restricted cytomegalovirus latency in the central nervous system regulated by CD4+ T-cells and IFN-γ
Journal of Neuroinflammation (2025)
-
Beyond hearing loss: exploring neurological and neurodevelopmental sequelae in asymptomatic congenital cytomegalovirus infection
Pediatric Research (2025)
-
A rhesus macaque model of congenital cytomegalovirus infection reveals a spectrum of vertical transmission outcomes
Communications Biology (2025)