Abstract
Background
The level and lactonase activity of paraoxonase 1 (PON1) and their association with PON1 genetic variants and oxidative stress are unclear in neonates of women with gestational diabetes mellitus (GDM).
Methods
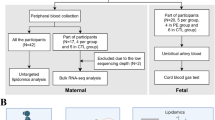
This study included 362 neonates of women with GDM and 302 control neonates. The level, lactonase activity, normalized lactonase activity (NLA), and genetic polymorphisms of PON1, serum total oxidant status (TOS), total antioxidant capacity (TAC), and malondialdehyde (MDA) were analyzed.
Results
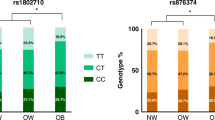
The neonates of the women with GDM had significantly higher levels, lactonase activity, and NLA of PON1, higher TOS, TAC, and MDA concentrations, and relatively higher oxidative stress index than those of the control neonates. The PON1 −108C → T variation decreased the lactonase activity, level, and NLA of PON1, while the PON1 192Q → R variation decreased the PON1 NLA in a genotype-dependent manner in the two groups. Multivariable regression analysis revealed the PON1 −108C/T or 192Q/R variation, apolipoprotein (apo)A1, or apoB as significant predictors of the level, lactonase activity, and NLA of PON1.
Conclusions
The lactonase activity, level, and NLA of PON1 were increased in the neonates of women with GDM. The PON1 genetic variants, abnormalities in lipoproteins, and increased oxidative stress may be associated with these changes.
Impact
-
This is the first study to report the elevated level, lactonase activity, and NLA of PON1 in the neonates of women with GDM.
-
These neonates also exhibited increased oxidative stress and an adverse glycolipid metabolic profile.
-
We further established that the −108C/T and/or 192Q/R genetic variants of the PON1 gene, abnormalities in lipoprotein metabolism, and/or increased oxidative stress had noticeable influences on the level and activities of PON1.
-
Whether these changes potentially cause metabolic disorders later in life remains to be determined. Therefore, the neonates born to women with GDM require further clinical follow-ups.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Caughey, A. B. & Turrentine, M. ACOG practice bulletin no. 190: Gestational diabetes mellitus. Obstet. Gynecol. 131, e49–e64 (2018).
Gao, C., Sun, X., Lu, L., Liu, F. & Yuan, J. Prevalence of gestational diabetes mellitus in mainland China: a systematic review and meta-analysis. J. Diabetes Investig. 10, 154–162 (2019).
Lowe, W. L. Jr, Scholtens, D. M., Sandler, V. & Hayes, M. G. Genetics of gestational diabetes mellitus and maternal metabolism. Curr. Diab. Rep. 16, 15 (2016).
Tam, W. H. et al. In utero exposure to maternal hyperglycemia increases childhood cardiometabolic risk in offspring. Diabetes Care 40, 679–686 (2017).
Bellamy, L., Casas, J. P., Hingorani, A. D. & Williams, D. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet 373, 1773–1779 (2009).
Ryckman, K. K., Spracklen, C. N., Smith, C. J., Robinson, J. G. & Saftlas, A. F. Maternal lipid levels during pregnancy and gestational diabetes: a systematic review and meta-analysis. Bjog 122, 643–651 (2015).
Zhang, C. et al. Genetic variants and the risk of gestational diabetes mellitus: a systematic review. Hum. Reprod. Update 19, 376–390 (2013).
Lopez-Tinoco, C. et al. Oxidative stress and antioxidant status in patients with late-onset gestational diabetes mellitus. Acta Diabetol. 50, 201–208 (2013).
Perla-Kajan, J. & Jakubowski, H. Paraoxonase 1 and homocysteine metabolism. Amino Acids 43, 1405–1417 (2012).
Precourt, L. P. et al. The three-gene paraoxonase family: physiologic roles, actions and regulation. Atherosclerosis 214, 20–36 (2011).
Camps, J., Marsillach, J. & Joven, J. The paraoxonases: role in human diseases and methodological difficulties in measurement. Crit. Rev. Clin. Lab. Sci. 46, 83–106 (2009).
Draganov, D. I. et al. Human paraoxonases (PON1, PON2, and PON3) are lactonases with overlapping and distinct substrate specificities. J. Lipid Res. 46, 1239–1247 (2005).
Rosenblat, M. et al. The catalytic histidine dyad of high density lipoprotein-associated serum paraoxonase-1 (PON1) is essential for PON1-mediated inhibition of low density lipoprotein oxidation and stimulation of macrophage cholesterol efflux. J. Biol. Chem. 281, 7657–7665 (2006).
Gugliucci, A. et al. Low protective PON1 lactonase activity in an Arab population with high rates of coronary heart disease and diabetes. Clin. Chim. Acta 445, 41–47 (2015).
Gaidukov, L. & Tawfik, D. S. The development of human sera tests for HDL-bound serum PON1 and its lipolactonase activity. J. Lipid Res. 48, 1637–1646 (2007).
Zhou, M. et al. Lactonase activity, status, and genetic variations of paraoxonase 1 in women with gestational diabetes mellitus. J. Diabetes Res. 2020, 3483427 (2020).
Wang, Y. et al. Evidence for association between paraoxonase 1 gene polymorphisms and polycystic ovarian syndrome in southwest Chinese women. Eur. J. Endocrinol. 166, 877–885 (2012).
Pappa, K. I., Gazouli, M., Anastasiou, E., Loutradis, D. & Anagnou, N. P. The Q192R polymorphism of the paraoxonase-1 (PON1) gene is associated with susceptibility to gestational diabetes mellitus in the Greek population. Gynecol. Endocrinol. 33, 617–620 (2017).
Erel, O. A new automated colorimetric method for measuring total oxidant status. Clin. Biochem. 38, 1103–1111 (2005).
Erel, O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin. Biochem. 37, 277–285 (2004).
Zhang, R. et al. Oxidative stress status in Chinese women with different clinical phenotypes of polycystic ovary syndrome. Clin. Endocrinol. 86, 88–96 (2017).
Shang, M., Zhao, J., Yang, L. & Lin, L. Oxidative stress and antioxidant status in women with gestational diabetes mellitus diagnosed by IADPSG criteria. Diabetes Res. Clin. Pr. 109, 404–410 (2015).
Camuzcuoglu, H., Toy, H., Cakir, H., Celik, H. & Erel, O. Decreased paraoxonase and arylesterase activities in the pathogenesis of future atherosclerotic heart disease in women with gestational diabetes mellitus. J. Women’s Health 18, 1435–1439 (2009).
International Association of Diabetes Pregnancy Study Groups Consensus, Panel International Association of Diabetes and Pregnancy Study Groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 33, 676–682 (2010).
Zhang, Y. et al. Lactonase activity and status of paraoxonase 1 in Chinese women with polycystic ovarian syndrome. Eur. J. Endocrinol. 172, 391–402 (2015).
Gao, Q. et al. Activity and distribution of plasma platelet-activating factor acetylhydrolase in women with gestational diabetes mellitus and their neonates. Diabetes Metab. Res. Rev. 32, 634–642 (2016).
Stefanovic, A. et al. Association of the atherogenic index of plasma and oxidative stress status with weight gain during non-complicated pregnancy. Clin. Chem. Lab. Med. 50, 2019–2025 (2012).
Stefanovic, A. et al. Longitudinal changes in PON1 activities, PON1 phenotype distribution and oxidative status throughout normal pregnancy. Reprod. Toxicol. 33, 20–26 (2012).
Lee, Y. H., Choi, S. H., Lee, K. W. & Kim, D. J. Apolipoprotein B/A1 ratio is associated with free androgen index and visceral adiposity and may be an indicator of metabolic syndrome in male children and adolescents. Clin. Endocrinol. 74, 579–586 (2011).
Sierra-Johnson, J. et al. Concentration of apolipoprotein B is comparable with the apolipoprotein B/apolipoprotein A-I ratio and better than routine clinical lipid measurements in predicting coronary heart disease mortality: findings from a multi-ethnic US population. Eur. Heart J. 30, 710–717 (2009).
Navab, M. et al. The oxidation hypothesis of atherogenesis: the role of oxidized phospholipids and HDL. J. Lipid Res. 45, 993–1007 (2004).
Barker, D. J. The Wellcome Foundation Lecture, 1994. The fetal origins of adult disease. Proc. Biol. Sci. 262, 37–43 (1995).
Khersonsky, O. & Tawfik, D. S. The histidine 115-histidine 134 dyad mediates the lactonase activity of mammalian serum paraoxonases. J. Biol. Chem. 281, 7649–7656 (2006).
Cole, T. B. et al. Expression of human paraoxonase (PON1) during development. Pharmacogenetics 13, 357–364 (2003).
Maciejczyk, M., Zalewska, A. & Ladny, J. R. Salivary antioxidant barrier, redox status, and oxidative damage to proteins and lipids in healthy children, adults, and the elderly. Oxid. Med. Cell Longev. 2019, 4393460 (2019).
Aviram, M. & Vaya, J. Paraoxonase 1 activities, regulation, and interactions with atherosclerotic lesion. Curr. Opin. Lipido. 24, 339–344 (2013).
Ardalic, D. et al. The influence of maternal smoking habits before pregnancy and antioxidative supplementation during pregnancy on oxidative stress status in a non-complicated pregnancy. Adv. Clin. Exp. Med. 23, 575–583 (2014).
James, R. W. & Deakin, S. P. The contribution of high density lipoprotein apolipoproteins and derivatives to serum paraoxonase-1 activity and function. Adv. Exp. Med. Biol. 660, 173–181 (2010).
Gaidukov, L. et al. ApoE induces serum paraoxonase PON1 activity and stability similar to ApoA-I. Biochemistry 49, 532–538 (2010).
Vekic, J. et al. Association of oxidative stress and PON1 with LDL and HDL particle size in middle-aged subjects. Eur. J. Clin. Invest. 37, 715–723 (2007).
Rosenblat, M., Karry, R. & Aviram, M. Paraoxonase 1 (PON1) is a more potent antioxidant and stimulant of macrophage cholesterol efflux, when present in HDL than in lipoprotein-deficient serum: relevance to diabetes. Atherosclerosis 187, 74–81 (2006).
Acknowledgements
We thank the women with GDM and the control group women who donated cord blood samples for this study. We also appreciate Qian Gao for participating in the sample collection. This study was supported by the Key Research and Development Project of Sichuan Province (grant no. 2019YFS0401), the National Key Research and Development Program of China (grant no. 2016YFC1000400) and the Program for Changjiang Scholars and Innovative Research Team in University, Ministry of Education (grant no. IRT0935).
Author information
Authors and Affiliations
Contributions
M.Z. collected samples, conducted experiments, and wrote the paper. P.F. designed the experiments, analyzed the data, and revised the paper. X.-H.L. and M.C. were responsible for patient screening and helped with the sample collection. Q.-Q.L., C.-Y.J., and L.-B.G. assisted with the experiments. H.B. helped with the data analysis of the results and revised the paper. All authors read and approved the final manuscript to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical statement
The present study was approved by the Institutional Review Board of the West China Second University Hospital, Sichuan University. All subjects provided their written informed consent in accordance with the Helsinki Declaration of ethical conduct in research.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Zhou, M., Liu, XH., Liu, QQ. et al. Lactonase activity and status of paraoxonase 1 and oxidative stress in neonates of women with gestational diabetes mellitus. Pediatr Res 89, 1192–1199 (2021). https://doi.org/10.1038/s41390-020-1023-2
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41390-020-1023-2
This article is cited by
-
Reduced low-density lipoprotein cholesterol levels are associated with increased risk of gestational diabetes mellitus in Chinese women
Scientific Reports (2025)
-
Association of xenobiotic-metabolizing gene cytochrome P450 2E1 variants with preeclampsia in Chinese women
Reproductive Sciences (2025)
-
Longitudinal changes of oxidative stress and PON1 lactonase activity and status in older pregnant women undergoing assisted reproductive technology: a prospective nested case-control study
Reproductive Biology and Endocrinology (2023)