Abstract
Background
Doxapram is used for the treatment of apnea of prematurity in dosing regimens only based on bodyweight, as pharmacokinetic data are limited. This study describes the pharmacokinetics of doxapram and keto-doxapram in preterm infants.
Methods
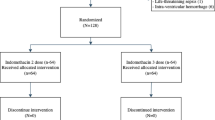
Data (302 samples) from 75 neonates were included with a median (range) gestational age (GA) 25.9 (23.9–29.4) weeks, bodyweight 0.95 (0.48–1.61) kg, and postnatal age (PNA) 17 (1–52) days at the start of continuous treatment. A population pharmacokinetic model was developed using non-linear mixed-effects modelling (NONMEM®).
Results
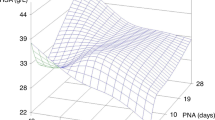
A two-compartment model best described the pharmacokinetics of doxapram and keto-doxapram. PNA and GA affected the formation clearance of keto-doxapram (CLFORMATION KETO-DOXAPRAM) and clearance of doxapram via other routes (CLDOXAPRAM OTHER ROUTES). For a median individual of 0.95 kg, GA 25.6 weeks, and PNA 29 days, CLFORMATION KETO-DOXAPRAM was 0.115 L/h (relative standard error (RSE) 12%) and CLDOXAPRAM OTHER ROUTES was 0.645 L/h (RSE 9%). Oral bioavailability was estimated at 74% (RSE 10%).
Conclusions
Dosing of doxapram only based on bodyweight results in the highest exposure in preterm infants with the lowest PNA and GA. Therefore, dosing may need to be adjusted for GA and PNA to minimize the risk of accumulation and adverse events. For switching to oral therapy, a 33% dose increase is required to maintain exposure.
Impact
-
Current dosing regimens of doxapram in preterm infants only based on bodyweight result in the highest exposure in infants with the lowest PNA and GA.
-
Dosing of doxapram may need to be adjusted for GA and PNA to minimize the risk of accumulation and adverse events.
-
Describing the pharmacokinetics of doxapram and its active metabolite keto-doxapram following intravenous and gastroenteral administration enables to include drug exposure to the evaluation of treatment of AOP.
-
The oral bioavailability of doxapram in preterm neonates is 74%, requiring a 33% higher dose via oral than intravenous administration to maintain exposure.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Poets, C. F. et al. Association between intermittent hypoxemia or bradycardia and late death or disability in extremely preterm infants. JAMA 314, 595–603 (2015).
Sawyer, T. et al. Improving neonatal intubation safety: a journey of a thousand miles. J. Neonatal Perinat. Med. 10, 125–131 (2017).
Sweet, D. G. et al. European Consensus Guidelines on the Management of Respiratory Distress Syndrome—2016 update. Neonatology 111, 107–125 (2017).
Morton, S. U. & Smith, V. C. Treatment options for apnoea of prematurity. Arch. Dis. Child Fetal Neonatal Ed. 101, F352–F356 (2016).
Schmidt, B. et al. Caffeine therapy for apnea of prematurity. N. Engl. J. Med. 354, 2112–2121 (2006).
Schmidt, B. et al. Survival without disability to age 5 years after neonatal caffeine therapy for apnea of prematurity. JAMA 307, 275–282 (2012).
Flint, R. et al. Retrospective study shows that doxapram therapy avoided the need for endotracheal intubation in most premature neonates. Acta Paediatr. 106, 733–739 (2017).
Prins, S. A. et al. Doxapram use for apnoea of prematurity in neonatal intensive care. Int. J. Pediatr. 2013, 251047 (2013).
Alpan, G. et al. Clinical and laboratory observations—doxapram in the treatment of idiopathic apnea of prematurity unresponsive to aminophylline. J. Pediatr. 104, 634–637 (1984).
Eyal, F. et al. Aminophylline versus doxapram in idiopathic apnea of prematurity—a double-blind controlled-study. Pediatrics 75, 709–713 (1985).
Dopram 2 mg/ml-actualisatie-SPC-08-2010. https://www.geneesmiddeleninformatiebank.nl/smpc/h07309_smpc.pdf. Revised 07-03-2012.
Barrington, K. J. et al. Dose–response relationship of doxapram in the therapy for refractory idiopathic apnea of prematurity. Pediatrics 80, 22–27 (1987).
Vliegenthart, R. J., Ten Hove, C. H., Onland, W. & van Kaam, A. H. Doxapram treatment for apnea of prematurity: a systematic review. Neonatology 111, 162–171 (2016).
Poets, C. F., Darraj, S. & Bohnhorst, B. Effect of doxapram on episodes of apnoea, bradycardia and hypoxaemia in preterm infants. Biol. Neonate 76, 207–213 (1999).
Peers, C. Effects of doxapram on ionic currents recorded in isolated type-i cells of the neonatal rat carotid-body. Brain Res. 568, 116–122 (1991).
Barrington, K. J., Finer, N. N., Peters, K. L. & Barton, J. Physiologic effects of doxapram in idiopathic apnea of prematurity. J. Pediatr. 108, 124–129 (1986).
Bairam, A. et al. Doxapram metabolism in human fetal hepatic organ culture. Clin. Pharmacol. Ther. 50, 32–38 (1991).
Ogawa, Y. et al. Population pharmacokinetics of doxapram in low-birth-weight Japanese infants with apnea. Eur. J. Pediatr. 174, 509–518 (2015).
Bairam, A. et al. Pharmacodynamic effects and pharmacokinetic profiles of keto-doxapram and doxapram in newborn lambs. Pediatr. Res. 28, 142–146 (1990).
Krekels, E. H. J. et al. Evidence-based drug treatment for special patient populations through model-based approaches. Eur. J. Pharm. Sci. 109S, S22–S26 (2017).
Brussee, J. M. et al. Children in clinical trials: towards evidence-based pediatric pharmacotherapy using pharmacokinetic-pharmacodynamic modeling. Expert Rev. Clin. Pharmacol. 9, 1235–1244 (2016).
De Cock, R. F. et al. The role of population PK-PD modelling in paediatric clinical research. Eur. J. Clin. Pharmacol. 67(Suppl. 1), 5–16 (2011).
Barbe, F. et al. Severe side effects and drug plasma concentrations in preterm infants treated with doxapram. Ther. Drug Monit. 21, 547–552 (1999).
Jamali, F. et al. Doxapram dosage regimen in apnea of prematurity based on pharmacokinetic data. Dev. Pharm. Ther. 11, 253–257 (1988).
Flint, R. B., Bahmany, S., van der Nagel, B. C. H. & Koch, B. C. P. Simultaneous quantification of fentanyl, sufentanil, cefazolin, doxapram and keto-doxapram in plasma using liquid chromatography—tandem mass spectrometry. Biomed. Chromatogr. 32, e4290 (2018).
Keizer, R. J., Karlsson, M. O. & Hooker, A. Modeling and simulation workbench for NONMEM: tutorial on Pirana, PsN, and Xpose. CPT Pharmacomet. Syst. Pharmacol. 2, e50 (2013).
Dosne, A. G., Bergstrand, M., Harling, K. & Karlsson, M. O. Improving the estimation of parameter uncertainty distributions in nonlinear mixed effects models using sampling importance resampling. J. Pharmacokinet. Pharmacodyn. 43, 583–596 (2016).
Lindbom, L., Pihlgren, P. & Jonsson, E. N. PsN-Toolkit—a collection of computer intensive statistical methods for non-linear mixed effect modeling using NONMEM. Comput. Methods Prog. Biomed. 79, 241–257 (2005).
Comets, E., Brendel, K. & Mentre, F. Computing normalised prediction distribution errors to evaluate nonlinear mixed-effect models: the npde add-on package for R. Comput. Methods Prog. Biomed. 90, 154–166 (2008).
Anchieta, L. M., Xavier, C. C., Colosimo, E. A. & Souza, M. F. Weight of preterm newborns during the first twelve weeks of life. Braz. J. Med. Biol. Res. 36, 761–770 (2003).
Greze, E. et al. Doxapram dosing for apnea of prematurity based on postmenstrual age and gender: a Randomized Controlled Trial. Paediatr. Drugs 18, 443–449 (2016).
Gow, P. J. et al. Neonatal hepatic drug elimination. Pharmacol. Toxicol. 88, 3–15 (2001).
Grijalva, J. & Vakili, K. Neonatal liver physiology. Semin. Pediatr. Surg. 22, 185–189 (2013).
Brussee, J. M. et al. Predicting CYP3A-mediated midazolam metabolism in critically ill neonates, infants, children and adults with inflammation and organ failure. Br. J. Clin. Pharmacol. 84, 358–368 (2018).
Voller, S. et al. Recently registered midazolam doses for preterm neonates do not lead to equal exposure: a population pharmacokinetic model. J. Clin. Pharmacol. 59, 1300–1308 (2019).
Robson, R. H. & Prescott, L. F. A pharmacokinetic study of doxapram in patients and volunteers. Br. J. Clin. Pharmacol. 7, 81–87 (1979).
Brussee, J. M. et al. First-pass CYP3A-mediated metabolism of midazolam in the gut wall and liver in preterm neonates. CPT Pharmacomet. Syst. Pharmacol. 7, 374–383 (2018).
Carroll, J. L. & Kim, I. Postnatal development of carotid body glomus cell O2 sensitivity. Respir. Physiol. Neurobiol. 149, 201–215 (2005).
Wong-Riley, M. T., Liu, Q. & Gao, X. P. Peripheral-central chemoreceptor interaction and the significance of a critical period in the development of respiratory control. Respir. Physiol. Neurobiol. 185, 156–169 (2013).
Poppe, J. A. et al. Precision dosing of doxapram in preterm infants using continuous pharmacodynamic data and model-based pharmacokinetics: an illustrative case series. Front. Pharmacol. 11, 665 (2020).
Maillard, C. et al. QT interval lengthening in premature infants treated with doxapram. Clin. Pharm. Ther. 70, 540–545 (2001).
De Villiers, G. S., Walele, A., Van der Merwe, P. L. & Kalis, N. N. Second-degree atrioventricular heart block after doxapram administration. J. Pediatr. 133, 149–150 (1998).
Tay-Uyboco, J. et al. Clinical and physiological responses to prolonged nasogastric administration of doxapram for apnea of prematurity. Biol. Neonate 59, 190–200 (1991).
Czaba-Hnizdo, C. et al. Amplitude-integrated electroencephalography shows that doxapram influences the brain activity of preterm infants. Acta Paediatr. 103, 922–927 (2014).
Fischer, C., Ferdynus, C., Gouyon, J. B. & Semama, D. S. Doxapram and hypokalaemia in very preterm infants. Arch. Dis. Child Fetal Neonatal Ed. 98, F416–F418 (2013).
Shimokaze, T., Toyoshima, K., Shibasaki, J. & Itani, Y. Blood potassium and urine aldosterone after doxapram therapy for preterm infants. J. Perinatol. 38, 702–707 (2018).
Flint, R. B. et al. Big data analyses for continuous evaluation of pharmacotherapy: a proof of principle with doxapram in preterm infants. Curr. Pharm. Des. 23, 5919–5927 (2017).
Acknowledgements
This study was enabled by funding from the Netherlands Organization for Health Research and Development ZonMw (Grant number: 80-83600-98-10190).
Author information
Authors and Affiliations
Consortia
Contributions
R.d.G. initiated the application of the grant that enabled the DINO study. R.B.F., S.H.P.S., R.d.G, D.M.B., and C.A.J.K. designed the study. P.L.J.D., P.A., K.D.L., I.K.M.R., and S.H.P.S. were local coordinating investigators and recruited subjects. B.C.P.K. facilitated the measurement of the plasma concentrations doxapram and keto-doxapram. R.B.F., A.G.J.E., R.t.H., C.A.J.K., and S.V. performed the population PK analyses. All authors have read and approved the final version of the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Patient consent
The Erasmus MC ethics review board approved the protocol and written informed consent from parents/legal guardians was obtained prior to study initiation (MEC-2014-067).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Flint, R.B., Simons, S.H.P., Andriessen, P. et al. The bioavailability and maturing clearance of doxapram in preterm infants. Pediatr Res 89, 1268–1277 (2021). https://doi.org/10.1038/s41390-020-1037-9
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-020-1037-9
This article is cited by
-
Oral doxapram for apnea of prematurity: A randomized dosage trial
Journal of Perinatology (2025)
-
Why population pharmacokinetic studies are instrumental to boost clinical research in neonates, and suggestions on how clinicians should assess these papers
Pediatric Research (2024)
-
When will the Glomerular Filtration Rate in Former Preterm Neonates Catch up with Their Term Peers?
Pharmaceutical Research (2024)
-
The effects of doxapram and its potential interactions with K2P channels in experimental model preparations
Journal of Comparative Physiology A (2024)
-
Doxapram versus placebo in preterm newborns: a study protocol for an international double blinded multicentre randomized controlled trial (DOXA-trial)
Trials (2023)