Abstract
Background
We investigated whether plasma high-sensitivity cardiac troponin T (hs-cTnT) and circulating heart-associated microRNA (miRs) are increased in children with leukaemias during anthracycline-based chemotherapeutic treatment.
Methods
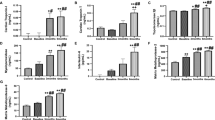
In vitro human pluripotent stem cell (hPSC)-derived cardiomyocyte model showed that miR-1, miR-133a, miR-208a, miR-208b, and miR-499 are released from cells into culture medium in a time- and dose-dependent manner on doxorubicin exposure. Left ventricular (LV) myocardial deformation and circulating heart-associated miRs and plasma hs-cTnT during and after completion of chemotherapy were determined in 40 children with newly diagnosed acute leukaemia.
Results
Significant reduction of LV global longitudinal strain and strain rates were found within 1 week after completion of anthracycline therapy in the induction phase of treatment (all p < 0.05). Hs-cTnT level peaked and miR-1 increased significantly at this time point. Log-transformed hs-cTnT correlated negatively with LV global systolic longitudinal strain (r = −0.38, p < 0.001). Receiver operating characteristic analysis revealed that area under the curve for changes in plasma hs-cTnT from baseline and plasma miR-1 levels in detecting a reduction in ≥20% of global longitudinal strain were respectively 0.62 (95% CI 0.38−0.87) and 0.62 (95% CI 0.40−0.84).
Conclusion
Plasma hs-cTnT and circulating miR-1 may be useful markers of myocardial damage during chemotherapy in children with leukaemias.
Impact
-
Heart-associated miRNAs including miR-1, miR-133a, miR-208a, miR-208b,and miR-499 were increased in the culture medium upon exposure of hPSC-derived cardiomyocytes to doxorubicin.
-
Only miR-1 increased significantly during anthracycline-based therapy in paediatric leukaemic patients.
-
In paediatric leukaemic patients, plasma hs-cTnT and circulating level of miR-1 showed the most significant increase within 1 week after completion of anthracycline therapy in the induction treatment phase.
-
The study provides the first evidence of progressive increase in circulating miR-1 and plasma hs-cTnT levels during the course of anthracycline-based therapy in children with leukaemias, with hs-cTnT level also associated with changes in LV myocardial deformation.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Kuwabara, Y. et al. Increased microRNA-1 and microRNA-133a levels in serum of patients with cardiovascular disease indicate myocardial damage. Circ. Cardiovasc. Genet. 4, 446–454 (2011).
Chow, M., Boheler, K. R. & Li, R. A. Human pluripotent stem cell-derived cardiomyocytes for heart regeneration, drug discovery and disease modeling: from the genetic, epigenetic, and tissue modeling perspectives. Stem Cell Res. Ther. 4, 97 (2013).
Mokuyasu, S., Suzuki, Y., Kawahara, E., Seto, T. & Tokuda, Y. High-sensitivity cardiac troponin I detection for 2 types of drug-induced cardiotoxicity in patients with breast cancer. Breast Cancer 22, 563–569 (2015).
Kitayama, H. et al. High-sensitive troponin T assay can predict anthracycline- and trastuzumab-induced cardiotoxicity in breast cancer patients. Breast Cancer 24, 774–782 (2017).
Leger, K. J. et al. Circulating microRNAs: potential markers of cardiotoxicity in children and young adults treated with anthracycline chemotherapy. J. Am. Heart Assoc. 6, e004653 (2017).
de Lemos, J. A. et al. Association of troponin T detected with a highly sensitive assay and cardiac structure and mortality risk in the general population. JAMA 304, 2503–2512 (2010).
Otsuka, T., Kawada, T., Ibuki, C. & Seino, Y. Association between high-sensitivity cardiac troponin T levels and the predicted cardiovascular risk in middle-aged men without overt cardiovascular disease. Am. Heart J. 159, 972–978 (2010).
Ai, J. et al. Circulating microRNA-1 as a potential novel biomarker for acute myocardial infarction. Biochem. Biophys. Res. Commun. 391, 73–77 (2010).
Cheng, Y. et al. A translational study of circulating cell-free microRNA-1 in acute myocardial infarction. Clin. Sci. 119, 87–95 (2010).
Kuwabara, Y. et al. Increased microRNA-1 and microRNA-133a levels in serum of patients with cardiovascular disease indicate myocardial damage. Circ. Cardiovasc. Genet. 4, 446–454 (2011).
Wang, G. K. et al. Circulating microRNA: a novel potential biomarker for early diagnosis of acute myocardial infarction in humans. Eur. Heart J. 31, 659–666 (2010).
Corsten, M. F. et al. Circulating MicroRNA-208b and MicroRNA-499 reflect myocardial damage in cardiovascular disease. Circ. Cardiovasc. Genet. 3, 499–506 (2010).
Adachi, T. et al. Plasma microRNA 499 as a biomarker of acute myocardial infarction. Clin. Chem. 56, 1183–1185 (2010).
Nishimura, Y. et al. Plasma miR-208 as a useful biomarker for drug-induced cardiotoxicity in rats. J. Appl. Toxicol. 35, 173–180 (2015).
Horie, T. et al. Acute doxorubicin cardiotoxicity is associated with miR-146a-induced inhibition of the neuregulin-ErbB pathway. Cardiovasc. Res. 87, 656–664 (2010).
Vacchi-Suzzi, C. et al. Perturbation of microRNAs in rat heart during chronic doxorubicin treatment. PLoS ONE 7, e40395 (2012).
Ambros, V. The functions of animal microRNAs. Nature 431, 350–355 (2004).
Liang, Y., Ridzon, D., Wong, L. & Chen, C. Characterization of microRNA expression profiles in normal human tissues. BMC Genomics 8, 166 (2007).
Mitchell, P. S. et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc. Natl. Acad. Sci. USA 105, 10513–10518 (2008).
Turchinovich, A., Weiz, L., Langheinz, A. & Burwinkel, B. Characterization of extracellular circulating microRNA. Nucleic Acids Res. 39, 7223–7233 (2011).
Cheung, Y. F., Hong, W. J., Chan, G. C., Wong, S. J. & Ha, S. Y. Left ventricular myocardial deformation and mechanical dyssynchrony in children with normal ventricular shortening fraction after anthracycline therapy. Heart 96, 1137–1141 (2010).
Cheung, Y. F., Li, S. N., Chan, G. C., Wong, S. J. & Ha, S. Y. Left ventricular twisting and untwisting motion in childhood cancer survivors. Echocardiography 28, 738–745 (2011).
Maillet, A. et al. Modeling doxorubicin-induced cardiotoxicity in human pluripotent stem cell derived-cardiomyocytes. Sci. Rep. 6, 25333 (2016).
Burridge, P. W. et al. Human induced pluripotent stem cell-derived cardiomyocytes recapitulate the predilection of breast cancer patients to doxorubicin-induced cardiotoxicity. Nat. Med. 22, 547–556 (2016).
Oliveira-Carvalho, V., Ferreira, L. R. & Bocchi, E. A. Circulating mir-208a fails as a biomarker of doxorubicin-induced cardiotoxicity in breast cancer patients. J. Appl. Toxicol. 35, 1071–1072 (2015).
Rigaud, V. O. et al. Circulating miR-1 as a potential biomarker of doxorubicin-induced cardiotoxicity in breast cancer patients. Oncotarget 8, 6994–7002 (2017).
Navickas, R. et al. Identifying circulating microRNAs as biomarkers of cardiovascular disease: a systematic review. Cardiovasc. Res. 111, 322–337 (2016).
Oatmen, K. E. et al. Identification of a novel microRNA profile in pediatric patients with cancer treated with anthracycline chemotherapy. Am. J. Physiol. Heart Circ. Physiol. 315, H1443–H1452 (2018).
Chistiakov, D. A., Orekhov, A. N. & Bobryshev, Y. V. Cardiac-specific miRNA in cardiogenesis, heart function, and cardiac pathology (with focus on myocardial infarction). J. Mol. Cell Cardiol. 94, 107–121 (2016).
Zhu, R. et al. Physical developmental cues for the maturation of human pluripotent stem cell-derived cardiomyocytes. Stem Cell Res. Ther. 5, 117 (2014).
Mathur, A. et al. Human iPSC-based cardiac microphysiological system for drug screening applications. Sci. Rep. 5, 8883 (2015).
Fu, J. D. et al. Distinct roles of microRNA-1 and -499 in ventricular specification and functional maturation of human embryonic stem cell-derived cardiomyocytes. PLoS ONE 6, e27417 (2011).
Wilson, K. D. et al. Dynamic microRNA expression programs during cardiac differentiation of human embryonic stem cells: role for miR-499. Circ. Cardiovasc. Genet. 3, 426–435 (2010).
Frost, B. M. et al. Pharmacokinetics of doxorubicin in children with acute lymphoblastic leukemia: multi-institutional collaborative study. Med. Pediatr. Oncol. 38, 329–337 (2002).
Cheung, Y. F. et al. Plasma high sensitivity troponin T levels in adult survivors of childhood leukaemias: determinants and associations with cardiac function. PLoS ONE 8, e77063 (2013).
Lipshultz, S. E. et al. Changes in cardiac biomarkers during doxorubicin treatment of pediatric patients with high-risk acute lymphoblastic leukemia: associations with long-term echocardiographic outcomes. J. Clin. Oncol. 30, 1042–1049 (2012).
Lipshultz, S. E. et al. Predictive value of cardiac troponin T in pediatric patients at risk for myocardial injury. Circulation 96, 2641–2648 (1997).
Thavendiranathan, P. et al. Use of myocardial strain imaging by echocardiography for the early detection of cardiotoxicity in patients during and after cancer chemotherapy: a systematic review. J. Am. Coll. Cardiol. 63, 2751–2768 (2014).
Oikonomou, E. K. et al. Assessment of prognostic value of left ventricular global longitudinal strain for early prediction of chemotherapy-induced cardiotoxicity: a systematic review and meta-analysis. JAMA Cardiol. https://doi.org/10.1001/jamacardio.2019.2952 (2019).
Armstrong, G. T. et al. Comprehensive echocardiographic detection of treatment-related cardiac dysfunction in adult survivors of childhood cancer: results from the St. Jude Lifetime Cohort Study. J. Am. Coll. Cardiol. 65, 2511–2522 (2015).
Acknowledgements
This study was supported by the Health and Health Services Research Fund, Food and Health Bureau, Hong Kong SAR Government (01121546).
Author information
Authors and Affiliations
Contributions
Y.-f.C. conceptualized the study, analysed the echocardiographic data and wrote the manuscript. V.W.-y.L. and C.T.-m.L. recruited the patients, acquired echocardiographic data and analysed the echocardiographic data. V.Y.S. and A.K. performed the circulating microRNA measurements and analysed laboratory results. W.K. and R.A.L. performed experiments on human pluripotent stem cell-derived cardiomyocytes and interpreted the in vitro data. D.K.-l.C. and G.C.-f.C. helped to recruit patients and analysed the clinical data. All of the authors had critically reviewed the manuscript, provided their intellectual input, and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Patient consent
Informed written consent was obtained from parents of all patients for this clinical study, which was approved by the Institutional Review Board and performed in accordance with the relevant guidelines and regulations.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Cheung, Yf., Li, V.Wy., Lai, C.Tm. et al. Circulating high-sensitivity troponin T and microRNAs as markers of myocardial damage during childhood leukaemia treatment. Pediatr Res 89, 1245–1252 (2021). https://doi.org/10.1038/s41390-020-1049-5
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-020-1049-5
This article is cited by
-
Long-term impact of anthracycline in early-stage breast cancer, bridging of MiRNAs profiler for early cardiotoxicity
Cardio-Oncology (2025)
-
Cardiotoxicity in pediatric oncology: a systematic review and meta-analysis
Pediatric Research (2025)
-
Exploring Predictive Risk Factors for Myocardial Injury in Children Treated with Anthracyclines: A Pilot Study
Cardiovascular Toxicology (2025)
-
Precision Cardio-oncology: Update on Omics-Based Diagnostic Methods
Current Treatment Options in Oncology (2024)
-
Acute and early-onset cardiotoxicity in children and adolescents with cancer: a systematic review
BMC Cancer (2023)