Abstract
Background
Previous studies have described an association between preterm birth and maturation of the autonomic nervous system (ANS); however, this may be impacted by multiple factors, including prematurity-related complications. Our aim was to evaluate for the effect of prematurity-related morbidity on ANS development in preterm infants in the NICU.
Methods
We compared time and frequency domains of heart rate variability (HRV) as a measure of ANS tone in 56 preterm infants from 2 NICUs (28 from each). One cohort was from a high-morbidity regional referral NICU, the other from a community-based inborn NICU with low prematurity-related morbidity. Propensity score matching was used to balance the groups by a 1:1 nearest neighbor design. ANS tone was analyzed.
Results
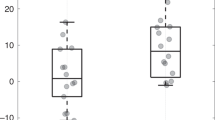
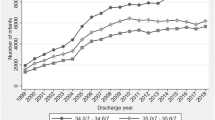
The two cohorts showed parallel maturational trajectory of the alpha 1 time-domain metric, with the cohort from the high-morbidity NICU having lower autonomic tone. The maturational trajectories between the two cohorts differed in all other time-domain metrics (alpha 2, RMS1, RMS2). There was no difference between groups by frequency-domain metrics.
Conclusions
Prematurity-associated morbidities correlate with autonomic development in premature infants and may have a greater impact on the extrauterine maturation of this system than birth gestational age.
Impact
-
Autonomic nervous system development measured by time-domain metrics of heart rate variability correlate with morbidities associated with premature birth.
-
This study builds upon our previously published work that showed that development of autonomic tone was not impacted by gestational age at birth.
-
This study adds to our understanding of autonomic nervous system development in a preterm extrauterine environment.
-
Our study suggests that gestational age at birth may have less impact on autonomic nervous system development than previously thought.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Mulkey, S. B. & du Plessis, A. J. Autonomic nervous system development and its impact on neuropsychiatric outcome. Pediatr. Res. 85, 120–126 (2019).
Siddiqui, S. et al. An antenatal marker of neurodevelopmental outcomes in infants with congenital heart disease. J. Perinatol. 37, 953–957 (2017).
Haraldsdottir, K. et al. Heart rate recovery after maximal exercise is impaired in healthy young adults born preterm. Eur. J. Appl. Physiol. 119, 857–866 (2019).
Thiriez, G. et al. Altered autonomic control in preterm newborns with impaired neurological outcomes. Clin. Auton. Res. 25, 233–242 (2015).
Mulkey, S. B. & Plessis, A. D. The critical role of the central autonomic nervous system in fetal-neonatal transition. Semin. Pediatr. Neurol. 28, 29–37 (2018).
Mulkey, S. B. et al. Autonomic nervous system depression at term in neurologically normal premature infants. Early Hum. Dev. 123, 11–16 (2018).
Yiallourou, S. R. et al. The development of autonomic cardiovascular control is altered by preterm birth. Early Hum. Dev. 89, 145–152 (2013).
Patural, H. et al. Autonomic cardiac control of very preterm newborns: a prolonged dysfunction. Early Hum. Dev. 84, 681–687 (2008).
Mulkey, S. B. et al. Autonomic nervous system maturation in the premature extrauterine milieu. Pediatr. Res. https://doi.org/10.1038/s41390-020-0952-0 (2020).
Ulusar, U. D. et al. Adaptive rule based fetal QRS complex detection using Hilbert transform. Conf. Proc. IEEE Eng. Med Biol. Soc. 1, 4666–4669 (2009).
Kota, S. et al. Identification of QRS complex in non-stationary electrocardiogram of sick infants. Comput. Biol. Med. 87, 211–216 (2017).
Govindan, R. B. et al. Detrended fluctuation analysis of non-stationary cardiac beat-to-beat interval of sick infants. EPL (Europhys. Lett.) 108, 40005 (2014).
Govindan, R. B. Detrended fluctuation analysis using orthogonal polynomials. Phys. Rev. E 101, 010201 (2020).
Govindan, R. B., Preissl, H., Eswaran, H., Campbell, J. Q. & Lowery, C. L. Detrended fluctuation analysis of short data sets: ana pplication to fetal cardiac. Phys. D Nonlinear Phenom. 226, 23–31 (2007).
Mulkey, S. B. et al. The effect of labor and delivery mode on electrocortical and brainstem autonomic function during neonatal transition. Sci. Rep. 9, 11020 (2019).
Fyfe, K. L. et al. The effect of gestational age at birth on post-term maturation of heart rate variability. Sleep 38, 1635–1644 (2015).
Karin, J., Hirsch, M. & Akselrod, S. An estimate of fetal autonomic state by spectral analysis of fetal heart rate fluctuations. Pediatr. Res. 34, 134–138 (1993).
Porges, S. W. in The Polyvagal Theory (ed. Schore, A. N.) 347 (WW Norton & Company, 2011).
Longin, E. et al. Maturation of the autonomic nervous system: differences in heart rate variability in premature vs. term infants. J. Perinat. Med. 34, 303–308 (2006).
Mulkey, S. B. et al. Heart rate variability is depressed in the early transitional period for newborns with complex congenital heart disease. Clin. Auton. Res. 30, 165–172 (2020).
Clairambault, J. et al. Heart rate variability in normal sleeping full-term and preterm neonates. Early Hum. Dev. 28, 169–183 (1992).
Barbe, M. F. & Levitt, P. The early commitment of fetal neurons to the limbic cortex. J. Neurosci. 11, 519–533 (1991).
DiPietro, J. A., Costigan, K. A. & Voegtline, K. M. Studies in fetal behavior: revisited, renewed, and reimagined. Monogr. Soc. Res. Child Dev. 80, vii–vii94 (2015).
DiPietro, J. A. et al. Fetal neurobehavioral development. Child Dev. 67, 2553–2567 (1996).
Lucchini, M. et al. Novel heart rate parameters for the assessment of autonomic nervous system function in premature infants. Physiol. Meas. 37, 1436–1446 (2016).
Malliani, A., Lombardi, F. & Pagani, M. Power spectrum analysis of heart rate variability: a tool to explore neural regulatory mechanisms. Br. Heart J. 71, 1–2 (1994).
Valenza, G. et al. Measures of sympathetic and parasympathetic autonomic outflow from heartbeat dynamics. J. Appl. Physiol. 125, 19–39 (2018).
Al-Shargabi, T. et al. Changes in autonomic tone in premature infants developing necrotizing enterocolitis. Am. J. Perinatol. 35, 1079–1086 (2018).
Joshi, R. et al. Predicting neonatal sepsis using features of heart rate variability, respiratory characteristics, and ECG-derived estimates of infant motion. IEEE J. Biomed. Health Inf. 24, 681–692 (2020).
Griffin, M. P. et al. Heart rate characteristics: novel physiomarkers to predict neonatal infection and death. Pediatrics 116, 1070–1074 (2005).
Griffin, M. P., Lake, D. E. & Moorman, J. R. Heart rate characteristics and laboratory tests in neonatal sepsis. Pediatrics 115, 937–941 (2005).
Vandenbroucke, L. et al. Chorioamnionitis following preterm premature rupture of membranes and fetal heart rate variability. PLoS ONE 12, e0184924 (2017).
Bohanon, F. J. et al. Heart rate variability analysis is more sensitive at identifying neonatal sepsis than conventional vital signs. Am. J. Surg. 210, 661–667 (2015).
Mulkey, S. B. et al. Heart rate variability is depressed in the early transitional period for newborns with complex congenital heart disease. Clin. Auton. Res. 30, 165–172 (2020).
Goudjil, S. et al. Patent ductus arteriosus in preterm infants is associated with cardiac autonomic alteration and predominant parasympathetic stimulation. Early Hum. Dev. 89, 631–634 (2013).
Metzler, M. et al. Pattern of brain injury and depressed heart rate variability in newborns with hypoxic ischemic encephalopathy. Pediatr. Res. 82, 438–443 (2017).
Massaro, A. N. et al. Heart rate variability in encephalopathic newborns during and after therapeutic hypothermia. J. Perinatol. 34, 836–841 (2014).
Reich, D. A. et al. The effect of unilateral stroke on autonomic function in the term newborn. Pediatr. Res. 85, 830–834 (2019).
Gomes, E. et al. Autonomic responses of premature newborns to body position and environmental noise in the neonatal intensive care unit. Rev. Bras. Ter. Intensiv. 31, 296–302 (2019).
Feldman, R., Rosenthal, Z. & Eidelman, A. I. Maternal-preterm skin-to-skin contact enhances child physiologic organization and cognitive control across the first 10 years of life. Biol. Psychiatry 75, 56–64 (2014).
Sanders, M. R. & Hall, S. L. Trauma-informed care in the newborn intensive care unit: promoting safety, security and connectedness. J. Perinatol. 38, 3–10 (2018).
Campbell, H. et al. Autonomic dysfunction in neonates with hypoxic ischemic encephalopathy undergoing therapeutic hypothermia impairs physiological responses to routine care events. J. Pediatr. 196, 38–44 (2018).
Nino, G. et al. Premature infants rehospitalized because of an apparent life-threatening event had distinctive autonomic developmental trajectories. Am. J. Respir. Crit. Care Med. 194, 379–381 (2016).
Kommers, D. R. et al. Features of heart rate variability capture regulatory changes during kangaroo care in preterm infants. J. Pediatr. 182, 92.e1–98.e1 (2017).
Feldman, R. & Eidelman, A. I. Skin-to-skin contact (Kangaroo Care) accelerates autonomic and neurobehavioural maturation in preterm infants. Dev. Med. Child Neurol. 45, 274–281 (2003).
Rand, K. & Lahav, A. Maternal sounds elicit lower heart rate in preterm newborns in the first month of life. Early Hum. Dev. 90, 679–683 (2014).
Horne, R. S. et al. Dummy/pacifier use in preterm infants increases blood pressure and improves heart rate control. Pediatr. Res. 79, 325–332 (2016).
Arnon, S. et al. Maternal singing during kangaroo care led to autonomic stability in preterm infants and reduced maternal anxiety. Acta Paediatr. 103, 1039–1044 (2014).
Acknowledgements
This study was supported by the Children’s National Inova Collaborative (CNICA) Research Program, through institutional support from Children’s National Hospital, Washington, DC and the Inova Health System, Fairfax, VA. S.B.M. received support by Award Numbers UL1TR001876 and KL2TR001877 from the NIH National Center for Advancing Translational Sciences. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
S.D.S. drafted the manuscript and participated in the study design, clinical data collection for the Children’s National cohort, and interpretation of study results. R.B.G. participated in the study design and data analysis and provided critical review of the manuscript. S.D.B. performed statistical analysis for the study and provided critical review of the manuscript. T.A.-S. collected data at INOVA, analyzed heart rate variability data, and provided critical review of the manuscript. D.A.R. and S.I. performed clinical data collection for the Children’s National cohort and provided critical review of the manuscript. L.H. was responsible for participant consent, enrollment, and clinical data collection for the INOVA cohort as well as providing critical review of the manuscript. G.L.M. and R.B. provided critical review of the manuscript. A.J.d.P. participated in the study design and critical review of the manuscript. S.B.M. designed the study, interpreted the study results, and provided critical review of the manuscript. All authors have reviewed and approved the final version of this manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent
Informed consent was obtained for subjects enrolled in our prospective study at Inova Fairfax Hospital, Fairfax, VA. Subjects at Children’s National Hospital were enrolled in a retrospective study with a waiver of informed consent.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Schlatterer, S.D., Govindan, R.B., Barnett, S.D. et al. Autonomic development in preterm infants is associated with morbidity of prematurity. Pediatr Res 91, 171–177 (2022). https://doi.org/10.1038/s41390-021-01420-x
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41390-021-01420-x
This article is cited by
-
Impact of Kangaroo mother care on autonomic cardiovascular control in foetal-growth-restricted preterm infants
Pediatric Research (2025)
-
Bedside tracking of functional autonomic age in preterm infants
Pediatric Research (2023)
-
Autonomic markers of extubation readiness in premature infants
Pediatric Research (2023)
-
Investigating the development of the autonomic nervous system in infancy through pupillometry
Journal of Neural Transmission (2023)
-
Vital signs as physiomarkers of neonatal sepsis
Pediatric Research (2022)