Abstract
Background
Modulation of behavior and physiology by dietary perturbations early in life can provide clues to the pathogenesis of adult diseases. We tested the hypothesis that a period of early protein supplementation modulates sympathetic nervous system activity demonstrated indirectly by an increase in active sleep state distribution in very low birth weight (VLBW) infants.
Methods
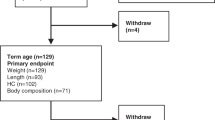
VLBW infants (n = 71) were randomized to a total parenteral nutritional regimen providing 18% of the energy intake as amino acids (AA) or a conventional regimen providing 12.5% to achieve targeted AA intakes of 4 g/kg/day (0.004 kcal/kg/day) and 3 g/kg/day (0.003 kcal/kg/day), respectively. Both groups were weaned to enteral feeding and advanced to provide similar AA intake of 4 g/kg/day (0.004 kcal/kg/day). Six-hour daytime, behavioral sleep studies were performed when the infants reached full enteral intake (165 ml/kg/day).
Results
Infants in the high protein group spent more time in active sleep (77.2 ± 10.5% vs. 70.7 ± 11.8%), p < 0.01 and less time in quiet sleep (12.9 ± 3.4% vs. 17.7 ± 7.0%, p < 0.01) as compared to the conventional group. No group differences were observed for indeterminate sleep, awake, or crying states.
Conclusions
These results suggest that dietary intake may indirectly influence sympathetic nervous system activity.
Impact
-
Infants randomized to an early, high protein nutritional regimen spent an increased percentage of time in active sleep, supporting the hypothesis that nutrition and behavior are interactive.
-
Furthermore, sleep states are an indirect measure of sympathetic nervous system activity, suggesting that dietary intake may influence sympathetic nervous system activity.
-
This study highlights the importance of considering the impact of nutrition during critical periods of development in order to further understand and improve the long-term outcomes of very low birth weight infants.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Lucas, A. Role of nutritional programming in determining adult morbidity. Arch. Dis. Child 71, 288–290 (1994).
Langley-Evans, S. C. Nutritional programming of disease: unravelling the mechanism. J. Anat. 215, 36–51 (2009).
Lucas, A. Does early diet program future outcome. Acta Paediatr. Scand. Suppl. 365, 58–61 (1990).
Cusick, S. & Georgieff, M. The role of nutrition in brain development: the golden opportunity of the “first 100 days”. J. Pediatr. 175, 16–21 (2016).
Pollitt, E., Gorman, K., Engle, P., Riveras, J., & Martorell, R. Nutrition in early life and the fulfillment of intellectual potential. J. Nutr. 125, 1111S–1118S (1995).
Pongcharoen, T. et al. Influence of prenatal and postnatal growth on intellectual functioning in school-aged children. Arch. Pediatr. Adolesc. Med. 166, 411–416 (2012).
Langley-Evans, S. C., Phillips, G. J. & Jackson, A. A. In utero exposure to maternal low protein diets induces hypertension in weanling rats, independently of maternal blood pressure changes. Clin. Nutr. 13, 319–324 (1994).
Ojeda, N. B., Grigore, D. & Alexander, B. T. Developmental programming of hypertension: insights from animal models of nutritional manipulation. Hypertension 52, 44–50 (2008).
DeKlerk, A. et al. Diet and infant behavior. Acta Pediatr. Suppl. 422, 65–68 (1997).
Georgieff, M., Ramel, S. & Cusick, S. Nutritional influences on brain development. Acta Pediatr. 107, 1310–1321 (2018).
Graven, S. Sleep and brain development. Clin. Perinatol. 33, 693–706 (2006).
Mirmiran, M. The importance of fetal/neonatal REM sleep. Eur. J. Obstet. Gynecol. Reprod. Gynecol. 21, 283–291 (1986).
Sahni, R., Schulze, K. F., Stefanski, M., Myers, M. M. & Fifer, W. P. Methodological issues in coding sleep states in immature infants. Dev. Psychobiol. 28, 85–101 (1995).
Mirmiran, M., Maas, Y. & Ariagno, R. Development of fetal and neonatal sleep and circadian rhythms. Sleep Med. Rev. 7, 321–334 (2002).
Barbeau, D. & Weiss, D. Sleep disturbances in newborns. Children 4, 90 (2017).
Butte, N., Jensen, C., Moon, J., Glaze, D. & Frost, J. Sleep organization and energy expenditure of breast fed and formula fed infants. Pediatr. Res. 32, 514–519 (1992).
Kashyap, S. et al. Effects of quality of energy intake on growth and metabolic response of enterally fed low-birth-weight infants. Pediatr. Res. 50, 390–397 (2001).
Dusick, A., Poindexter, B., Ehrenkranz, R. & Lemons, J. Growth failure in the preterm infant: can we catch up? Semin. Perinatol. 27, 302–310 (2003).
Cetin, I. et al. Umbilical amino acid concentrations in normal and growth-retarded fetuses sampled in utero by cordocentesis. Am. J. Obstet. Gynecol. 162, 253–261 (1990).
Stefanski, M. et al. A scoring system for states of sleep and wakefulness in term and preterm infants. Pediatr. Res. 18, 58–62 (1984).
Masterson, J., Zucker, C. & Schulze, K. Prone supine positioning effects on energy expenditure behavior of low birth weight infants. Pediatrics 80, 689–692 (1987).
Myers, M. M. et al. Effects of sleeping position time after feeding on the organization of sleep/wake states in prematurely born infants. Sleep 21, 343–349 (1998).
Brackbill, Y., Douthitt, T. C. & West, H. Psychophysiologic effects in the neonate of prone versus supine placement. J. Pediatr. 82, 82–84 (1973).
Hashimoto, T. et al. Postural effects on behavioral states of newborn infants: a sleep polygraphic study. Brain Dev. 5, 286–291 (1983).
Drucker-Colin, R., Spanis, C. W., Cotman, C. W. & McGaugh, J. L. Changes in protein level in perfusates of freely moving cats: relation to behavioral states. Science 187, 963–965 (1997).
Danguir, J. & Nicholaidis, S. Intravenous infusion of nutrients and sleep in the rat. Anischymetric sleep regulation hypothesis. Am. Physiol. 238, E307–E312 (1980).
Pegram, V., Hammond, D. & Bridgers, W. The effects of protein synthesis inhibition on sleep in mice. Behav. Biol. 9, 377–382 (1973).
Macfayden, U. M., Oswald, L. & Lewis, S. A. Starvation and human slow-wave sleep. J. Appl. Physiol. 35, 391–394 (1973).
Oswald, I. Human brain proteins, drugs and dreams. Nature 223, 893–897 (1969).
Harper, A. E., Benevenga, N. J. & Wohlhueter, R. M. Effects of ingestion of disproportionate amounts of amino acids. Physiol. Rev. 50, 428–558 (1970).
Peters, J. C. & Harper, A. E. Protein and energy consumption, plasma amino acids ratio, and brain neurotransmitter concentration. Physiol. Behav. 27, 287–298 (1981).
Jouvet, M. Indolamines and sleep-inducing factors. Exp. Brain Res. Suppl. 8, 81–94 (1984).
Yogman, M. W. & Zeisel, S. H. Diet and sleep patterns in newborn infants. N. Engl. J. Med. 309, 1147–1149 (1983).
Hortensius, L. M. et al. Postnatal nutrition to improve brain development in the preterm infant: a systematic review from bench to bedside. Front. Physiol. 10, 96178 (2019).
Marchi, V. et al. Measuring cot-side the effects of parenteral nutrition on preterm cortical function. Front. Hum. Neurosci. 14, 69–78 (2020).
Shellhaas, R., Burns, J., Barks, J. & Chervin, R. Quantitative sleep stage analyses as a window to neonatal neurologic function. Neurology 82, 390–395 (2014).
Shellhaas, R. et al. Neonatal sleep-wake analyses predict 18-month neurodevelopmental outcomes. Sleep 40, 144 (2017).
Scher, M., Steppe, D. & Banks, D. Prediction of lower developmental performances on healthy neonates by neonatal EEG sleep measures. Pediatr. Neurol. 14, 137–144 (1996).
Acknowledgements
This study was supported by the NIH R01 HD27564 and National Center for Advancing Translational Sciences, National Institutes of Health, through Grant Number 1UL1TR001873-01. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Author information
Authors and Affiliations
Contributions
Each author has met the Pediatric Research authorship requirements.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Statement of Consent
Informed consent was obtained from the parent or guardian of each participant in the study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hammond, J., Kamboj, R., Kashyap, S. et al. The interaction between diet and neurobehavior in very low birth weight infants. Pediatr Res 91, 646–651 (2022). https://doi.org/10.1038/s41390-021-01464-z
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-021-01464-z