Abstract
Background
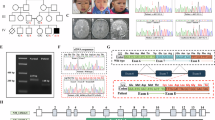
Lethal neonatal rigidity and multifocal seizure syndrome (RMFSL) is caused by variants in BRAT1 (BRCA1-associated protein required for ATM activation-1). However, the molecular mechanism of RMFSL is still unclear.
Methods
An RMFSL infant was recruited and the peripheral blood samples from his trio-family were collected. The genomic DNA was extracted, and then the whole-exome sequencing was performed. The expression of BRAT1 was analyzed by Western blotting. The subcellular localization of BRAT1 and MitoSOX (mitochondrial superoxide level) was investigated by confocal microscopy. The RNA samples were obtained from transfected cells, and then the RNA sequencing was performed.
Results
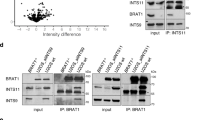
In this study, a novel homozygous BRAT1 variant c.233G > C with amino acid change of R with P at residue 78 (R78P) was identified. This variant altered the peptide structure and subcellular localization, as well as the expression in vitro. However, R78P did not alter the ability of BRAT1 to downregulate MitoSOX in mitochondria. Meanwhile, R78P BRAT1 was positively correlated with temporal lobe epilepsy, autosomal recessive primary microcephaly, defective/absent horizontal voluntary eye movements, and neuron apoptotic process as indicated by gene set enrichment analysis (GSEA).
Conclusions
The BRAT1 variant spectrum has been expanded, which will be helpful for genetic counseling. We also explored the molecular mechanism altered by R78P, which will provide a better understanding of the pathogenesis of RMFSL.
Impact
-
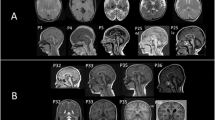
The detailed course of an infant with lethal neonatal RMFSL was depicted.
-
A novel disease-causing variant R78P in BRAT1 for lethal neonatal RMFSL was identified.
-
R78P led to reduced BRAT1 expression and nuclear localization in vitro.
-
R78P did not alter the ability of BRAT1 to downregulate MitoSOX in the mitochondria.
-
The variant R78P in BRAT1 was positively correlated with temporal lobe epilepsy, autosomal recessive primary microcephaly, defective/absent horizontal voluntary eye movements, and neuron apoptotic process as indicated by GSEA.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Puffenberger, E. G. et al. Genetic mapping and exome sequencing identify variants associated with five novel diseases. PLoS ONE 7, e28936 (2012).
So, E. Y. & Ouchi, T. BRAT1 deficiency causes increased glucose metabolism and mitochondrial malfunction. BMC Cancer 14, 548 (2014).
van de Pol, L. A. et al. Early-onset severe encephalopathy with epilepsy: the BRAT1 gene should be added to the list of causes. Neuropediatrics 46, 392–400 (2015).
Saitsu, H. et al. Compound heterozygous BRAT1 mutations cause familial Ohtahara syndrome with hypertonia and microcephaly. J. Hum. Genet. 59, 687–690 (2014).
Straussberg, R. et al. Lethal neonatal rigidity and multifocal seizure syndrome—report of another family with a BRAT1 mutation. Eur. J. Paediatr. Neurol. 19, 240–242 (2015).
Hanes, I., Kozenko, M. & Callen, D. J. A. Lethal neonatal rigidity and multifocal seizure syndrome—a misnamed disorder? Pediatr. Neurol. 53, 535–540 (2015).
Srivastava, S. & Naidu, S. Epileptic encephalopathy due to BRAT1 pathogenic variants. Pediatr. Neurol. Briefs 30, 45 (2016).
Fernández-Jaén, A. et al. Mutations in BRAT1 cause autosomal recessive progressive encephalopathy: report of a Spanish patient. Eur. J. Paediatr. Neurol. 20, 421–425 (2016).
Celik, Y., Okuyaz, C., Arslankoylu, A. E. & Ceylaner, S. Lethal neonatal rigidity and multifocal seizure syndrome with a new mutation in BRAT1. Epilepsy Behav. Case Rep. 8, 31–32 (2017).
Van Ommeren, R. H., Gao, A. F., Blaser, S. I., Chitayat, D. A. & Hazrati, L. BRAT1 mutation: the first reported case of Chinese origin and review of the literature. J. Neuropathol. Exp. Neurol. 77, 1071–1078 (2018).
Szymanska, K. et al. Clinico-pathological correlation in case of BRAT1 mutation. Folia Neuropathol. 56, 362–371 (2018).
Valence, S. et al. Exome sequencing in congenital ataxia identifies two new candidate genes and highlights a pathophysiological link between some congenital ataxias and early infantile epileptic encephalopathies. Genet. Med. 21, 553–563 (2019).
Oatts, J. T., Duncan, J. L., Hoyt, C. S., Slavotinek, A. M. & Moore, A. T. Inner retinal dystrophy in a patient with biallelic sequence variants in BRAT1. Ophthalmic Genet. 38, 559–561 (2017).
Horn, D. et al. BRAT1 mutations are associated with infantile epileptic encephalopathy, mitochondrial dysfunction, and survival into childhood. Am. J. Med. Genet. A 170, 2274–2281 (2016).
Smith, N. J., Lipsett, J., Dibbens, L. M. & Heron, S. E. BRAT1-associated neurodegeneration: intra-familial phenotypic differences in siblings. Am. J. Med. Genet. A 170, 3033–3038 (2016).
Mundy, S. A., Krock, B. L., Mao, R. & Shen, J. J. BRAT1-related disease—identification of a patient without early lethality. Am. J. Med. Genet. A 170, 699–702 (2016).
Srivastava, S. et al. BRAT1 mutations present with a spectrum of clinical severity. Am. J. Med. Genet. A 170, 2265–2273 (2016).
Scheffer, I. E. et al. BRAT1 encephalopathy: a recessive cause of epilepsy of infancy with migrating focal seizures. Dev. Med. Child Neurol. 62, 1096–1099 (2019).
Mahjoub, A. et al. Homozygous pathogenic variant in BRAT1 associated with nonprogressive cerebellar ataxia. Neurol. Genet. 5, e359 (2019).
Ouchi, M. & Ouchi, T. Regulation of ATM/DNA-PKcs phosphorylation by BRCA1-associated BAAT1. Genes Cancer 1, 1211–1214 (2010).
Aglipay, J. A., Martin, S. A., Tawara, H., Lee, S. W. & Ouchi, T. ATM activation by ionizing radiation requires BRCA1-associated BAAT1. J. Biol. Chem. 281, 9710–9718 (2006).
So, E. Y. & Ouchi, T. The potential role of BRCA1-associated ATM activator-1 (BRAT1) in regulation of mTOR. J. Cancer Biol. Res. 1, 3 (2013).
Zhang, C. J. et al. The Arabidopsis acetylated histone-binding protein BRAT1 forms a complex with BRP1 and prevents transcriptional silencing. Nat. Commun. 7, 11715 (2016).
Choi, J. Y. et al. CIDE domains form functionally important higher-order assemblies for DNA fragmentation. Proc. Natl Acad. Sci. USA 114, 7361–7366 (2017).
Lugovskoy, A. A., Zhou, P., Chou, J. J., McCarty, J. S. & Wagner, G. Solution structure of the CIDE-N domain of CIDE-B and a model for CIDE-N/CIDE-N interactions in the DNA fragmentation pathway of apoptosis. Cell 99, 747–755 (1999).
Acknowledgements
We would like to thank the family for joining in the study. We thank Jin Ma for the technical support. This work was supported by the National Natural Science Foundation of China (Nos. 81803116, 81570455, 81870365, and 81570125) for work designing and whole-exome sequencing, the Basic Research Project for Medical and Health Applications of Suzhou City (Nos. SYS2018077, SYS2019083, and SYS201757) for reagent purchasing, the Jiangsu Province’s Science Technology Support Program (Social Development) Project (BE2017658) and Startup fund (Q410800320) from Soochow University for data collection, and Jiangsu Province Medical Key Talents (ZDRCA2016049) for analyzing data and writing the report.
Author information
Authors and Affiliations
Contributions
W.L. and X. Zhao were responsible for the diagnosis and treatment of the patient. Y.P. analyzed the EEG results. H.H. analyzed the RNA-seq results. H.L., X. Zhu, H.X., and Y.L. designed the project. W.L., S.W., and H.X. performed the experiments. Y.L. wrote the manuscript and H.X. revised it.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Statement of consent
Written consent was obtained from the proband’s parent.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Li, W., Wu, S., Xu, H. et al. Novel variant in BRAT1 with the lethal neonatal rigidity and multifocal seizure syndrome. Pediatr Res 91, 565–571 (2022). https://doi.org/10.1038/s41390-021-01468-9
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-021-01468-9
This article is cited by
-
Clinical characteristics of BRAT1-related disease: a systematic literature review
Acta Neurologica Belgica (2024)
-
BRAT1 links Integrator and defective RNA processing with neurodegeneration
Nature Communications (2022)