Abstract
Background
Therapeutic hypothermia is a standard of care for neonatal encephalopathy; however, approximately one in two newborn infants fails to respond to this treatment. Recent studies have suggested potential relationships between body temperature, heart rate and the outcome of cooled infants.
Methods
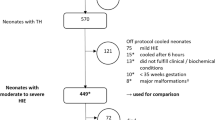
The clinical data of 756 infants registered to the Baby Cooling Registry of Japan between January 2012 and December 2016 were analysed to assess the relationship between body temperature, heart rate and adverse outcomes (death or severe impairment at 18 months corrected age).
Results
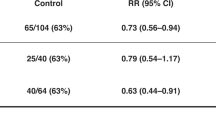
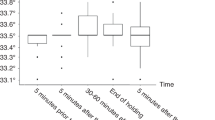
A lower body temperature at admission was associated with adverse outcomes in the univariate analysis (P < 0.001), the significance of which was lost when adjusted for the severity of encephalopathy and other covariates. A higher body temperature during cooling and higher heart rate before and during cooling were associated with adverse outcomes in both univariate (all P < 0.001) and multivariate (P = 0.012, P < 0.001 and P < 0.001, respectively) analyses.
Conclusions
Severe hypoxia–ischaemia might be a common causative of faster heart rates before and during cooling and low body temperature before cooling, whereas causal relationships between slightly higher temperatures during cooling and adverse outcomes need to be elucidated in future studies.
Impact
-
In a large cohort of encephalopathic newborn infants, dual roles of body temperature to the outcome were shown; adverse outcomes were associated with a lower body temperature at admission and higher body temperature during cooling.
-
A higher heart rate before and during cooling were associated with adverse outcomes.
-
Severe hypoxia–ischaemia might be a common causative of faster heart rates before and during cooling and low body temperature before cooling.
-
The exact mechanism underlying the relationship between slightly higher body temperature during cooling and adverse outcomes remains unknown, which needs to be elucidated in future studies.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Perlman, J. M. et al. Part 7: Neonatal Resuscitation: 2015 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations. Circulation 132, S204–S241 (2015).
Jacobs, S. E. et al. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst. Rev. 1, CD003311 (2013).
Shankaran, S. et al. Childhood outcomes after hypothermia for neonatal encephalopathy. N. Engl. J. Med. 366, 2085–2092 (2012).
Azzopardi, D. et al. Effects of hypothermia for perinatal asphyxia on childhood outcomes. N. Engl. J. Med. 371, 140–149 (2014).
Pappas, A. et al. Hypocarbia and adverse outcome in neonatal hypoxic-ischemic encephalopathy. J. Pediatr. 158, 752–758 e751 (2011).
Wyatt, J. S. et al. Determinants of outcomes after head cooling for neonatal encephalopathy. Pediatrics 119, 912–921 (2007).
Rao, R. et al. Safety and short-term outcomes of therapeutic hypothermia in preterm neonates 34-35 weeks gestational age with hypoxic-ischemic encephalopathy. J. Pediatr. 183, 37–42 (2017).
Basu, S. K. et al. Hypoglycaemia and hyperglycaemia are associated with unfavourable outcome in infants with hypoxic ischaemic encephalopathy: a post hoc analysis of the CoolCap Study. Arch. Dis. Child. Fetal Neonatal Ed. 101, F149–F155 (2016).
Laptook, A. R. et al. Elevated temperature and 6- to 7-year outcome of neonatal encephalopathy. Ann. Neurol. 73, 520–528 (2013).
Davidson, J. O. et al. How long is too long for cerebral cooling after ischemia in fetal sheep? J. Cereb. Blood Flow Metab. 35, 751–758 (2015).
Gunn, A. J. et al. Dramatic neuronal rescue with prolonged selective head cooling after ischemia in fetal lambs. J. Clin. Investig. 99, 248–256 (1997).
Gunn, A. J. et al. Neuroprotection with prolonged head cooling started before postischemic seizures in fetal sheep. Pediatrics 102, 1098–1106 (1998).
Sabir, H., Scull-Brown, E., Liu, X. & Thoresen, M. Immediate hypothermia is not neuroprotective after severe hypoxia-ischemia and is deleterious when delayed by 12 h in neonatal rats. Stroke 43, 3364–3370 (2012).
Tsuda, K. et al. Body temperature, heart rate, and short-term outcome of cooled infants. Ther. Hypothermia Temp. Manag. 9, 76–85 (2019).
Staer-Jensen, H. et al. Bradycardia during therapeutic hypothermia is associated with good neurologic outcome in comatose survivors of out-of-hospital cardiac arrest. Crit. Care Med. 42, 2401–2408 (2014).
Elstad, M., Liu, X. & Thoresen, M. Heart rate response to therapeutic hypothermia in infants with hypoxic-ischaemic encephalopathy. Resuscitation 106, 53–57 (2016).
Montaldo, P. et al. Electrocardiographic and echocardiographic changes during therapeutic hypothermia in encephalopathic infants with long-term adverse outcome. Resuscitation 130, 99–104 (2018).
Laptook, A. R. et al. Effect of therapeutic hypothermia initiated after 6 h of age on death or disability among newborns with hypoxic-ischemic encephalopathy: a randomized clinical trial. JAMA 318, 1550–1560 (2017).
Thoresen, M. et al. Time is brain: starting therapeutic hypothermia within three hours after birth improves motor outcome in asphyxiated newborns. Neonatology 104, 228–233 (2013).
Edwards, A. D. et al. Neurological outcomes at 18 months of age after moderate hypothermia for perinatal hypoxic ischaemic encephalopathy: synthesis and meta-analysis of trial data. BMJ 340, c363 (2010).
Tagin, M. A. et al. Hypothermia for neonatal hypoxic ischemic encephalopathy: an updated systematic review and meta-analysis. Arch. Pediatr. Adolesc. Med. 166, 558–566 (2012).
Wood, T. et al. Rectal temperature in the first five hours after hypoxia-ischaemia critically affects neuropathological outcomes in neonatal rats. Pediatr. Res. 83, 536–544 (2018).
Shankaran, S. et al. Effect of depth and duration of cooling on deaths in the NICU among neonates with hypoxic ischemic encephalopathy: a randomized clinical trial. JAMA 312, 2629–2639 (2014).
Shankaran, S. et al. Effect of depth and duration of cooling on death or disability at age 18 months among neonates with hypoxic-ischemic encephalopathy: a randomized clinical trial. JAMA 318, 57–67 (2017).
Sarnat, H. B. & Sarnat, M. S. Neonatal encephalopathy following fetal distress. A clinical and electroencephalographic study. Arch. Neurol. 33, 696–705 (1976).
Thompson, C. M. et al. The value of a scoring system for hypoxic ischaemic encephalopathy in predicting neurodevelopmental outcome. Acta Paediatr. 86, 757–761 (1997).
Palisano, R. et al. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev. Med. Child Neurol. 39, 214–223 (1997).
Kendall, G. S. et al. Passive cooling for initiation of therapeutic hypothermia in neonatal encephalopathy. Arch. Dis. Child. Fetal Neonatal Ed. 95, F408–F412 (2010).
Kämäräinen, A. et al. Induction of therapeutic hypothermia during prehospital CPR using ice-cold intravenous fluid. Resuscitation 79, 205–211 (2008).
Lemyre, B. et al. Initiation of passive cooling at referring centre is most predictive of achieving early therapeutic hypothermia in asphyxiated newborns. Paediatr. Child Health 22, 264–268 (2017).
Roberts, C. T., Stewart, M. J. & Jacobs, S. E. Earlier initiation of therapeutic hypothermia by non-tertiary neonatal units in Victoria, Australia. Neonatology 110, 33–39 (2016).
Wood, S. C. & Gonzales, R. Hypothermia in hypoxic animals: mechanisms, mediators, and functional significance. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 113, 37–43 (1996).
Enweronu-Laryea, C. et al. Core temperature after birth in babies with neonatal encephalopathy in a sub-Saharan African hospital setting. J. Physiol. 597, 4013–4024 (2019).
Hallberg, B. et al. Passive induction of hypothermia during transport of asphyxiated infants: a risk of excessive cooling. Acta Paediatr. 98, 942–946 (2009).
Szakmar, E. et al. Asphyxiated neonates who received active therapeutic hypothermia during transport had higher rates of hypocapnia than controls. Acta Paediatr. 107, 1902–1908 (2018).
Iwata, O. et al. Depth of delayed cooling alters neuroprotection pattern after hypoxia-ischemia. Ann. Neurol. 58, 75–87 (2005).
Gluckman, P. D. et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet 365, 663–670 (2005).
Shankaran, S. et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N. Engl. J. Med. 353, 1574–1584 (2005).
Azzopardi, D. V. et al. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N. Engl. J. Med. 361, 1349–1358 (2009).
Takenouchi, T., Iwata, O., Nabetani, M. & Tamura, M. Therapeutic hypothermia for neonatal encephalopathy: JSPNM & MHLW Japan Working Group Practice Guidelines Consensus Statement from the Working Group on Therapeutic Hypothermia for Neonatal Encephalopathy, Ministry of Health, Labor and Welfare (MHLW), Japan, and Japan Society for Perinatal and Neonatal Medicine (JSPNM). Brain Dev. 34, 165–170 (2012).
Thoresen, M. & Whitelaw, A. Cardiovascular changes during mild therapeutic hypothermia and rewarming in infants with hypoxic-ischemic encephalopathy. Pediatrics 106, 92–99 (2000).
Davies, P. & Maconochie, I. The relationship between body temperature, heart rate and respiratory rate in children. Emerg. Med. J. 26, 641–643 (2009).
Kreimeier, U. Pathophysiology of fluid imbalance. Crit. Care 4, S3–S7 (2000).
Nestaas, E. & Walsh, B. H. Hypothermia and cardiovascular instability. Clin. Perinatol. 47, 575–592 (2020).
Sarkar, S. et al. Esophageal and rectal temperatures as estimates of core temperature during therapeutic whole-body hypothermia. J. Pediatr. 162, 208–210 (2013).
Tsuda, K. et al. Therapeutic hypothermia for neonatal encephalopathy: a report from the first 3 years of the Baby Cooling Registry of Japan. Sci. Rep. 7, 39508 (2017).
Shibasaki, J. et al. Outcomes related to 10-min Apgar scores of zero in Japan. Arch. Dis. Child. Fetal Neonatal Ed. 105, 64–68 (2020).
Acknowledgements
The authors are grateful to the staff of participating centres for their contribution to the data collection, the infants and their parents for sharing the clinical information. This work was supported by the Japan Society of Perinatal and Neonatal Medicine, and the Ministry of Health, Labour and Welfare, Japan (H27-001, Special research in perinatal medicine). K.T. is funded by the Japan Science and Technology Agency and the Ministry of Education, Culture, Sports, Science and Technology (Grant-in-Aid for Scientific Research 18K15722). J.S. was funded by the Japan Science and Technology Agency and the Ministry of Education, Culture, Sports, Science and Technology (Grant-in-Aid for Scientific Research C20K08247). S.I. was funded by the Japan Science and Technology Agency and the Ministry of Education, Culture, Sports, Science and Technology (Grant-in-Aid for Scientific Research C18K09955). O.I. was funded by the Japan Science and Technology Agency and the Ministry of Education, Culture, Sports, Science and Technology (Grant-in-Aid for Scientific Research A20H00102).
Author information
Authors and Affiliations
Consortia
Contributions
K.T., S.I., M.N. and O.I. designed the study and the survey items. All authors participated in the data collection. K.T., T. Isayama, S.I. and O.I. performed the statistical analyses. K.T., J.S., T. Isayama, A.T., T.M. and O.I. contributed to the interpretation of the findings. K.T., J.S. and O.I. drafted the manuscript. All authors critically reviewed and revised the manuscript and gave the final approval of the published version. All authors agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent statement
The ethics committees approved that parental consent was not needed for the registry, since no patient identifier was collected.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Tsuda, K., Shibasaki, J., Isayama, T. et al. Body temperature, heart rate and long-term outcome of cooled infants: an observational study. Pediatr Res 91, 921–928 (2022). https://doi.org/10.1038/s41390-021-01502-w
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-021-01502-w
This article is cited by
-
Association between cardiovascular care and neurodevelopmental outcomes in infants with neonatal encephalopathy and hemodynamic instability
Journal of Perinatology (2025)
-
Three-year outcome following neonatal encephalopathy in a high-survival cohort
Scientific Reports (2022)
-
Admission temperature of very low birth weight infants and outcomes at three years old
Scientific Reports (2022)