Abstract
Background
Enriched language exposure may benefit infants in the neonatal intensive care unit. We hypothesized that changes in neonatal electroencephalogram (EEG) coherence during sleep, in response to maternal voice exposure, predict language development.
Methods
Convalescent neonates underwent 12-h polysomnography. A recording of the mother’s voice was randomized to continuous playback in the first or second 6 h. We calculated the imaginary coherence (ICOH—a measure of functional connectivity) between EEG leads. Spearman correlations were computed between ICOH and 18-month Bayley-III language scores.
Results
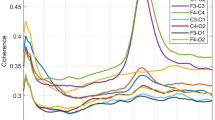
Thirty-five neonates were included (N = 18 33-to-<35 weeks gestation; N = 17 ≥ 35 weeks). Predictive value of ICOH during neonatal non-rapid eye movement (NREM) sleep was left lateralized, and varied with gestational age and voice playback. ICOH in the left-hemispheric (C3-Cz; T3-Cz) channels across multiple EEG frequency bands was associated with 18-month language scores (rho = −0.34 to −0.48). The association was driven by neonates born at 33–34 weeks gestation, and a trend suggested a possible effect of maternal voice at some EEG frequencies. Right hemisphere ICOH (C4-Cz; T4-Cz) was not associated with language outcome.
Conclusions
Left-hemispheric EEG functional connectivity during neonatal NREM sleep shows early signs of physiologic asymmetry that may predict language development. We speculate that sleep analyses could have unique prognostic value.
Impact
-
During neonatal NREM sleep, EEG functional connectivity predicts future language development.
-
Left temporal and central EEG coherence—specifically the imaginary component of coherence—is predictive, whereas the same analysis from the right hemisphere is not.
-
These results appear to vary according to the infant’s gestational age, and a trend suggests they may be enhanced by measuring functional connectivity during exposure to the mother’s voice.
-
These findings identify early evidence of physiologic differentiation within the cerebral hemispheres and raise the possibility that neonatal NREM sleep has a role to play in language development.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Matthews, L. G. et al. Brain growth in the NICU: critical periods of tissue-specific expansion. Pediatr. Res. 83, 976–981 (2018).
White, R. D. Designing environments for developmental care. Clin. Perinatol. 38, 745–749 (2011).
White, R. D. Recommended standards for newborn ICU design, 9th edition. J. Perinatol. 40, 2–4 (2020) .
Pineda, R. G. et al. Alterations in brain structure and neurodevelopmental outcome in preterm infants hospitalized in different neonatal intensive care unit envrionments. J. Pediatr. 164, 52–60 (2014).
de Villers-Sidani, E., Chang, E. F., Bao, S. & Merzenich, M. M. Critcal period window for spetral tuning defned in the primay auditory cortex (A1) in the rat. J. Neurosci. 27, 180–189 (2007).
Mowery, T. M., Kotak, V. C. & Sanes, D. H. Transient hearing loss within a critical period causes persistent changes to cellular properties in adult auditory cortex. Cereb. Cortex 25, 2083–2094 (2015).
Oliver, D. L., Izquierdo, M. A. & Malmierca, M. S. Persistent effects of early augmented acoustic environment on the auditory brainstem. Neuroscience 184, 75–87 (2011).
Bose, M. et al. Effect of the environment on the dendritic morphology of the rat auditory cortex. Synapase 64, 97–110 (2010).
Monson, B. B. et al. Differential rates of perinatal maturation of human primary and nonprimary auditory cortex. eNeuro 5 e0380-17.2017 1–12 (2018).
Shellhaas, R. A., Burns, J. W., Barks, J. D. E., Hassan, F. & Chervin, R. D. Maternal voice and infant sleep in the neonatal intensive care unit. Pediatrics 144 (2019).
Shellhaas, R. A. et al. Neonatal sleep-wake analyses predict 18-month neurodevelopmental outcomes. Sleep 40, zsx144 (2017).
Meijer, E. J. et al. Functional connectivity in preterm infants derived from EEG coherence analysis. Eur. J. Paediatr. Neurol. 18, 780–789 (2014).
González, J. J. et al. Assessment of electroencephalographic functional connectivity in term and preterm neonates. Clin. Neurophysiol. 122, 696–702 (2011).
McLaren, J., Holmes, G. L. & Berg, M. T. Functional connectivity in term neonates with hypoxic-ischemic encephalopathy undergoing therapeutic hypothermia. Pediatr. Neurol. 94, 74–79 (2019).
McAnulty, G. et al. School-age effects of the newborn individualized developmental care and assessment program for preterm infants with intrauterine growth restriction: preliminary findings. BMC Pediatr. 13, 25 (2013).
Myers, M. M. et al. Family Nurture Intervention in preterm infants alters frontal cortical functional connectivity assessed by EEG coherence. Acta Paediatr. 104, 670–677 (2015).
American Electroencephalographic Society guidelines for standard electrode position nomenclature. J. Clin. Neurophysiol. 8, 200–202 (1991).
Shellhaas, R. A. et al. The American Clinical Neurophysiology Society’s guideline on continuous electroencephalography monitoring in neonates. J. Clin. Neurophysiol. 28, 611–617 (2011).
Fox, M. Time for Bed: HMH Books for Young Readers (HMH, 1997).
Wood, A. The Napping House: HMH Books for Young Readers (HMH, 2015).
Welch, P. D. The use of fast Fourier transform for the estimation of power spectra: a method based on time averaging over short, modified periodograms. IEEE Trans. Audio Electroacoust. AU-15, 70–73 (1967).
Nolte, G. et al. Identifying true brain interaction from EEG data using the imaginary part of coherency. Clin. Neurophysiol. 115, 2292–2307 (2004).
Wirth, L. et al. Effects of standardized acoustic stimulation in premature infants: a randomized controlled trial. J. Perinatol. 36, 486–492 (2016).
Kaffashi, F., Scher, M. S., Ludington-Hoe, S. M. & Loparo, K. A. An analysis of the kangaroo care intervention using neonatal EEG complexity: a preliminary study. Clin. Neurophysiol. 124, 238–246 (2013).
Levy, J. et al. Impact of hands-on care on infant sleep in the neonatal intensive care unit. Pediatr. Pulmonol. 52, 84–90 (2017).
Acknowledgements
The authors deeply appreciate the infants and parents for their interest in research and enthusiasm for this work. They also thank the clinical research coordinators and sleep technologists, whose tireless attention to detail and care for each of the participating infants was integral to this study. This study was supported by NIH and the Michigan Institute for Clinical and Health Research (R21HD083409; UL1TR002240).
Author information
Authors and Affiliations
Contributions
R.A.S. conceptualized and designed the study, acquired and analyzed the data, drafted the initial manuscript, and approved the final version for submission. R.D.C. assisted with study design and interpretation of the results, critically reviewed the manuscript for important intellectual content, and approved the final version for submission. J.D.E.B. assisted with study design and interpretation of the results, critically reviewed the manuscript for important intellectual content, and approved the final version for submission. F.H interpreted the research polysomnograms, assisted with interpretation of the data, critically reviewed the manuscript for important intellectual content, and approved the final version for submission. M.D.C. assisted with interpretation of the data, critically reviewed the manuscript for important intellectual content, and approved the final version for submission. J.W.B. analyzed the data, assisted with interpretation of the data, critically reviewed the manuscript for important intellectual content, and approved the final version for submission.
Corresponding author
Ethics declarations
Competing interests
R.A.S’ research is supported by grants from NIH, PCORI, the Pediatric Epilepsy Research Foundation, and the University of Michigan. She serves as a consultant for the Epilepsy Study Consortium and as an Associate Editor for Neurology. She receives royalties from UpToDate for authorship of topics related to neonatal seizures. R.D.C. has had financial relationships with the American Academy of Sleep Medicine, the Associated Professional Sleep Societies, UpToDate, and Cambridge University Press; has been a member of the boards for the International Pediatric Sleep Association and the not-for-profit Sweet Dreamzzz; is a member of an advisory board for the not-for-profit Pajama Program; receives research support from the NIH; is named in patents and copyrighted material, owned by the University of Michigan, that concern identification and treatment of sleep disorders; and has received royalties from Zansors. J.D.E.B. receives grant funding from NIH. F.H. has previously served as a consultant for Biogen, and has received research funding from Jazz pharmaceuticals. M.D.C. has nothing to disclose. J.W.B. receives grant funding from NIH and is named in patents, owned by Michigan Technological University and the University of Michigan, that concern identification and treatment of sleep disorders.
Consent statement
A parent of every infant provided written informed consent to participate in the study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Shellhaas, R.A., Chervin, R.D., Barks, J.D.E. et al. Lateralized neonatal EEG coherence during sleep predicts language outcome. Pediatr Res 91, 962–969 (2022). https://doi.org/10.1038/s41390-021-01554-y
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41390-021-01554-y
This article is cited by
-
QEEG findings in nonsyndromic sagittal craniosynostosis
Scientific Reports (2024)