Abstract
Background
Preterm neonates often require glucocorticoids to manage refractory hypotension, prevent, and treat bronchopulmonary dysplasia. We have investigated the effect of cumulative dose and duration of glucocorticoids on blood pressure and renal function in VLBW infants.
Methods
In this retrospective cohort study, medical records of infants (GA ≤ 35 weeks) born January 2015 to December 2019 were reviewed to extract demographic and clinical characteristics, dose and duration of steroids, blood pressure (BP), and creatinine at the time of discharge from the neonatal intensive care unit.
Results
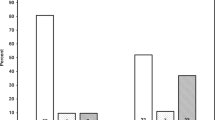
Two hundred and eighty-three neonates with average GA (28 ± 3 weeks) and birthweight (1060±381 g). Twenty-eight percent (33/116) of infants who received postnatal steroids developed hypertension versus 16% (27/167) of controls (OR = 2.0, p = 0.011). There was a correlation between the cumulative dosage of postnatal steroids and systolic BP (R2 = 0.06, p < 0.001). With increasing steroid dose and total steroid days, there was a significant increase in creatinine clearance at the time of discharge (R2 = 0.13, p < 0.001; R2 = 0.13, p < 0.001, respectively).
Conclusions
Cumulative dose of postnatal steroids and duration of use is associated with increased systolic BP in premature infants. Postnatal steroids should be used prudently to prevent long-term cardiovascular and renal morbidity.
Impact
-
Preterm neonates are exposed to a high dose of glucocorticoids during their neonatal intensive care stay.
-
The dose and duration of use of postnatal glucocorticoids was associated with significant increase in blood pressure at the time of discharge in preterm neonates.
-
Postnatal glucocorticoid use is associated with improved creatinine clearance likely due to a state of hyperfiltration and may lead to chronic kidney disease later in life.
-
Postnatal glucocorticoids should be used prudently in this highly vulnerable population
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Allen, M. C., Cristofalo, E. A. & Kim, C. Outcomes of preterm infants: morbidity replaces mortality. Clin. Perinatol. 38, 441–454 (2011).
Crump, C., Sundquist, K., Sundquist, J. & Winkleby, M. A. Gestational age at birth and mortality in young adulthood. JAMA 306, 1233–1240 (2011).
Jenkins, R. D., Aziz, J. K., Gievers, L. L., Mooers, H. M., Fino, N. & Rozansky, D. J. et al. Characteristics of hypertension in premature infants with and without chronic lung disease: a long-term multi-center study. Pediatr. Nephrol. 32, 2115–2124 (2017).
Abman, S. H., Warady, B. A., Lum, G. M. & Koops, B. L. Systemic hypertension in infants with bronchopulmonary dysplasia. J. Pediatr. 104, 928–931 (1984).
Anderson, A. H., Warady, B. A., Daily, D. K., Johnson, J. A. & Thomas, M. K. Systemic hypertension in infants with severe bronchopulmonary dysplasia: associated clinical factors. Am. J. Perinatol. 10, 190–193 (1993).
Alagappan, A. & Malloy, M. H. Systemic hypertension in very low-birth weight infants with bronchopulmonary dysplasia: incidence and risk factors. Am. J. Perinatol. 15, 3–8 (1998).
Abitbol, C. L. & Rodriguez, M. M. The long-term renal and cardiovascular consequences of prematurity. Nat. Rev. Nephrol. 8, 265-74-74 (2012).
Abitbol, C. L., Bauer, C. R., Montané, B., Chandar, J., Duara, S. & Zilleruelo, G. Long-term follow-up of extremely low birth weight infants with neonatal renal failure. Pediatr. Nephrol. 18, 887–893 (2003).
Fanos, V., Gerosa, C., Loddo, C. & Faa, G. State of the art on kidney development: how nephron endowment at birth can shape our susceptibility to renal dysfunction later in life. Am. J. Perinatol. 36, S33–S36 (2019).
Baum, M. Role of the kidney in the prenatal and early postnatal programming of hypertension. Am. J. Physiol. Ren. Physiol. 298, F235–247 (2010).
Mackenzie, H. S., Lawler, E. V. & Brenner, B. M. Congenital oligonephropathy: the fetal flaw in essential hypertension? Kidney Int. Suppl. 55, S30–S34 (1996).
Keller, G., Zimmer, G., Mall, G., Ritz, E. & Amann, K. Nephron number in patients with primary hypertension. N. Engl. J. Med. 348, 101–108 (2003).
Shankaran, S., Das, A., Bauer, C. R., Bada, H., Lester, B. & Wright, L. et al. Fetal origin of childhood disease: intrauterine growth restriction in term infants and risk for hypertension at 6 years of age. Arch. Pediatr. Adolesc. Med. 160, 977–981 (2006).
Ortiz, L. A., Quan, A., Weinberg, A. & Baum, M. Effect of prenatal dexamethasone on rat renal development. Kidney Int. 59, 1663–1669 (2001).
Dickinson, H., Walker, D. W., Wintour, E. M. & Moritz, K. Maternal dexamethasone treatment at midgestation reduces nephron number and alters renal gene expression in the fetal spiny mouse. Am. J. Physiol. Regul. Integr. Comp. Physiol. 292, R453–R461 (2007).
Chan, S. K., Riley, P. R., Price, K. L., McElduff, F., Winyard, P. J. & Welham, S. J. et al. Corticosteroid-induced kidney dysmorphogenesis is associated with deregulated expression of known cystogenic molecules, as well as Indian hedgehog. Am. J. Physiol. Ren. Physiol. 298, F346–356 (2010).
Greenough, A., Emery, E. F. & Gamsu, H. R. Dexamethasone and hypertension in preterm infants. Eur. J. Pediatr. 151, 134–135 (1992).
Smets, K. & Vanhaesebrouck, P. Dexamethasone associated systemic hypertension in low birth weight babies with chronic lung disease. Eur. J. Pediatr. 155, 573–575 (1996).
Flynn, JT et al. Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics 140, e20171904 (2017).
American Academy of Pediatrics Committee on Fetus and Newborn: routine evaluation of blood pressure, hematocrit, and glucose in newborns. Pediatrics 92, 474–476 (1993).
Jobe, A. H. & Bancalari, E. Bronchopulmonary dysplasia. Am. J. Respir. Crit. Care Med. 163, 1723–1729 (2001).
Miller, W. L. & Fluck, C. E. Pediatric Endocrinology (Elsevier Saunders, 2014).
Dionne, J. M., Abitbol, C. L. & Flynn, J. T. Hypertension in infancy: diagnosis, management and outcome. Pediatr. Nephrol. 27, 17–32 (2012).
Schwartz, G. J., Haycock, G. B., Edelmann, C. M. Jr. & Spitzer, A. A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics 58, 259–263 (1976).
Valinsky, W. C., Touyz, R. M. & Shrier, A. Aldosterone, SGK1, and ion channels in the kidney. Clin. Sci. (Lond.) 132, 173–183 (2018).
Molina-Jijón, E., Rodríguez-Muñoz, R., González-Ramírez, R., Namorado-Tónix, C., Pedraza-Chaverri, J. & Reyes, J. L. Aldosterone signaling regulates the over-expression of claudin-4 and -8 at the distal nephron from type 1 diabetic rats. PLoS ONE 12, e0177362 (2017).
Seliem, W. A., Falk, M. C., Shadbolt, B. & Kent, A. L. Antenatal and postnatal risk factors for neonatal hypertension and infant follow-up. Pediatr. Nephrol. 22, 2081–2087 (2007).
Blowey, D. L., Duda, P. J., Stokes, P. & Hall, M. Incidence and treatment of hypertension in the neonatal intensive care unit. J. Am. Soc. Hypertens. 5, 478–483 (2011).
Kraut, E. J., Boohaker, L. J., Askenazi, D. J., Fletcher, J. & Kent, A. L. Incidence of neonatal hypertension from a large multicenter study [Assessment of Worldwide Acute Kidney Injury Epidemiology in Neonates-AWAKEN]. Pediatr. Res. 84, 279–289 (2018).
Stow, L. R., Voren, G. E., Gumz, M. L., Wingo, C. S. & Cain, B. D. Dexamethasone stimulates endothelin-1 gene expression in renal collecting duct cells. Steroids 77, 360–366 (2012).
Itani, O. A., Liu, K. Z., Cornish, K. L., Campbell, J. R. & Thomas, C. P. Glucocorticoids stimulate human sgk1 gene expression by activation of a GRE in its 5’-flanking region. Am. J. Physiol. Endocrinol. Metab. 283, E971–979 (2002).
Hines, E. R., Collins, J. F., Jones, M. D., Serey, S. H. & Ghishan, F. K. Glucocorticoid regulation of the murine PHEX gene. Am. J. Physiol. Ren. Physiol. 283, F356–363 (2002).
Keller-Wood, M., von Reitzenstein, M. & McCartney, J. Is the fetal lung a mineralocorticoid receptor target organ? Induction of cortisol-regulated genes in the ovine fetal lung, kidney and small intestine. Neonatology 95, 47–60 (2009).
Menendez-Castro, C., Nitz, D., Cordasic, N., Jordan, J., Bäuerle, T. & Fahlbusch, F. B. et al. Neonatal nephron loss during active nephrogenesis—detrimental impact with long-term renal consequences. Sci. Rep. 8, 4542 (2018).
Seckl, J. R. Prenatal glucocorticoids and long-term programming. Eur. J. Endocrinol. 151, U49–62 (2004).
Zhang, J., Massmann, G. A., Rose, J. C. & Figueroa, J. P. Differential effects of clinical doses of antenatal betamethasone on nephron endowment and glomerular filtration rate in adult sheep. Reprod. Sci. 17, 186–195 (2010).
Moritz, K. M., De Matteo, R., Dodic, M., Jefferies, A. J., Arena, D. & Wintour, E. M. et al. Prenatal glucocorticoid exposure in the sheep alters renal development in utero: implications for adult renal function and blood pressure control. Am. J. Physiol. Regul. Integr. Comp. Physiol. 301, R500–509 (2011).
Wintour, E. M., Moritz, K. M., Johnson, K., Ricardo, S., Samuel, C. S. & Dodic, M. et al. Reduced nephron number in adult sheep, hypertensive as a result of prenatal glucocorticoid treatment. J. Physiol. 549, 929–935 (2003).
Singh, R. R., Cullen-McEwen, L. A., Kett, M. M., Boon, W. M., Dowling, J. & Bertram, J. F. et al. Prenatal corticosterone exposure results in altered AT1/AT2, nephron deficit and hypertension in the rat offspring. J. Physiol. 579, 503–513 (2007).
Acknowledgements
This study was internally funded by Department of Pediatrics, MetroHealth Medical Center, Case Western Reserve University, Cleveland, OH.
Author information
Authors and Affiliations
Contributions
C.M. and H.K. were responsible for conducting study, data collection, data analysis and writing initial draft of manuscript. M.P., N.K., R.R., P.P., and C.M. were responsible for data analysis and preparing tables and figures and helped write the initial draft of the manuscript. P.S. was responsible for study design, data analysis, data interpretation and wrote the final version of the manuscript. All authors approved the final revised version of submitted manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
This retrospective chart review study involving human subjects was in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The Human Investigation Review Committee (IRB) of MetroHealth Medical Center approved this study # IRB19-00229.
Consent statement
This was a retrospective study and IRB granted waiver of consent from subjects.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mhanna, C., Pinto, M., Koechley, H. et al. Postnatal glucocorticoid use impacts renal function in VLBW neonates. Pediatr Res 91, 1821–1826 (2022). https://doi.org/10.1038/s41390-021-01624-1
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-021-01624-1
This article is cited by
-
Hydrocortisone
Reactions Weekly (2022)