Abstract
Background
Docosahexaenoic acid (DHA) and arachidonic acid (AA) are important for fetal brain growth and development. Our aim was to evaluate the association between serum DHA and AA levels and brain volumes in extremely preterm infants.
Methods
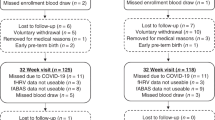
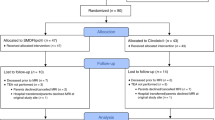
Infants born at <28 weeks gestational age in 2013–2015, a cohort derived from a randomized controlled trial comparing two types of parenteral lipid emulsions, were included (n = 90). Serum DHA and AA levels were measured at postnatal days 1, 7, 14, and 28, and the area under the curve was calculated. Magnetic resonance (MR) imaging was performed at term-equivalent age (n = 66), and volumes of six brain regions were automatically generated.
Results
After MR image quality assessment and area under the curve calculation, 48 infants were included (gestational age mean [SD] 25.5 [1.4] weeks). DHA levels were positively associated with total brain (B = 7.966, p = 0.012), cortical gray matter (B = 3.653, p = 0.036), deep gray matter (B = 0.439, p = 0.014), cerebellar (B = 0.932, p = 0.003), and white matter volume (B = 3.373, p = 0.022). AA levels showed no association with brain volumes.
Conclusions
Serum DHA levels during the first 28 postnatal days were positively associated with volumes of several brain structures in extremely preterm infants at term-equivalent age.
Impact
-
Higher serum levels of DHA in the first 28 postnatal days are positively associated with brain volumes at term-equivalent age in extremely preterm born infants.
-
Especially the most immature infants suffer from low DHA levels in the first 28 postnatal days, with little increase over time.
-
Future research is needed to explore whether postnatal fatty acid supplementation can improve brain development and may serve as a nutritional preventive and therapeutic treatment option in extremely preterm infants.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Martinez, M. Tissue levels of polyunsaturated fatty acids during early human development. J. Pediatr. 120, S129–S138 (1992).
Mallick, R., Basak, S. & Duttaroy, A. K. Docosahexaenoic acid,22:6n-3: Its roles in the structure and function of the brain. Int. J. Dev. Neurosci. 79, 21–31 (2019).
Lauritzen, L., Hansen, H. S., Jorgensen, M. H. & Michaelsen, K. F. The essentiality of long chain n-3 fatty acids in relation to development and function of the brain and retina. Prog. Lipid Res. 40, 1–94 (2001).
Hadley, K. B., Ryan, A. S., Forsyth, S., Gautier, S. & Salem, N. Jr. The essentiality of arachidonic acid in infant development. Nutrients 8, 216 (2016).
De Rooy, L., Hamdallah, H. & Dyall, S. C. Extremely preterm infants receiving standard care receive very low levels of arachidonic and docosahexaenoic acids. Clin. Nutr. 36, 1593–1600 (2017).
Lapillonne, A., Eleni dit Trolli, S. & Kermorvant-Duchemin, E. Postnatal docosahexaenoic acid deficiency is an inevitable consequence of current recommendations and practice in preterm infants. Neonatology 98, 397–403 (2010).
Martin, C. R. et al. Decreased postnatal docosahexaenoic and arachidonic acid blood levels in premature infants are associated with neonatal morbidities. J. Pediatr. 159, 743–749.e741–742 (2011).
Lapillonne, A. & Jensen, C. L. Reevaluation of the DHA requirement for the premature infant. Prostaglandins Leukot. Essent. Fatty Acids 81, 143–150 (2009).
Baack, M. L., Puumala, S. E., Messier, S. E., Pritchett, D. K. & Harris, W. S. What is the relationship between gestational age and docosahexaenoic acid (DHA) and arachidonic acid (ARA) levels? Prostaglandins Leukot. Essent. Fatty Acids 100, 5–11 (2015).
Lapillonne, A. et al. The use of low-EPA fish oil for long-chain polyunsaturated fatty acid supplementation of preterm infants. Pediatr. Res. 48, 835–841 (2000).
Lapillonne, A., Brossard, N., Claris, O., Reygrobellet, B. & Salle, B. L. Erythrocyte fatty acid composition in term infants fed human milk or a formula enriched with a low eicosapentanoic acid fish oil for 4 months. Eur. J. Pediatr. 159, 49–53 (2000).
Hellstrom, A. et al. Docosahexaenoic acid and arachidonic acid levels are associated with early systemic inflammation in extremely preterm infants. Nutrients 12, 1996 (2020).
Khwaja, O. & Volpe, J. J. Pathogenesis of cerebral white matter injury of prematurity. Arch. Dis. Child Fetal Neonatal Ed. 93, F153–F161 (2008).
Kamino, D. et al. Postnatal polyunsaturated fatty acids associated with larger preterm brain tissue volumes and better outcomes. Pediatr. Res. 83, 93–101 (2018).
Keunen, K. et al. Brain tissue volumes in preterm infants: prematurity, perinatal risk factors and neurodevelopmental outcome: a systematic review. J. Matern. Fetal Neonatal Med. 25(Suppl. 1), 89–100 (2012).
Keunen, K. et al. Brain volumes at term-equivalent age in preterm infants: imaging biomarkers for neurodevelopmental outcome through early school age. J. Pediatr. 172, 88–95 (2016).
Najm, S. et al. Effects of a lipid emulsion containing fish oil on polyunsaturated fatty acid profiles, growth and morbidities in extremely premature infants: a randomized controlled trial. Clin. Nutr. ESPEN 20, 17–23 (2017).
Lofqvist, C. A. et al. Association of retinopathy of prematurity with low levels of arachidonic acid: a secondary analysis of a randomized clinical trial. JAMA Ophthalmol. 136, 271–277 (2018).
Hansen-Pupp, I. et al. Influence of insulin-like growth factor I and nutrition during phases of postnatal growth in very preterm infants. Pediatr. Res. 69, 448–453 (2011).
Bockmann, K. A. et al. Fatty acid composition of adipose tissue at term indicates deficiency of arachidonic and docosahexaenoic acid and excessive linoleic acid supply in preterm infants. Eur. J. Nutr. 60, 861–872 (2021).
Papile, L. A., Burstein, J., Burstein, R. & Koffler, H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J. Pediatr. 92, 529–534 (1978).
Kidokoro, H., Neil, J. J. & Inder, T. E. New MR imaging assessment tool to define brain abnormalities in very preterm infants at term. AJNR Am. J. Neuroradiol. 34, 2208–2214 (2013).
Makropoulos, A. et al. The developing human connectome project: a minimal processing pipeline for neonatal cortical surface reconstruction. Neuroimage 173, 88–112 (2018).
Gousias, I. S. et al. Magnetic resonance imaging of the newborn brain: manual segmentation of labelled atlases in term-born and preterm infants. Neuroimage 62, 1499–1509 (2012).
Makropoulos, A. et al. Automatic whole brain MRI segmentation of the developing neonatal brain. IEEE Trans. Med. Imaging 33, 1818–1831 (2014).
Niklasson, A. et al. An update of the Swedish reference standards for weight, length and head circumference at birth for given gestational age (1977–1981). Acta Paediatr. Scand. 80, 756–762 (1991).
Express Group. Incidence of and risk factors for neonatal morbidity after active perinatal care: extremely preterm infants study in Sweden (EXPRESS). Acta Paediatr. 99, 978–992 (2010).
Express Group. et al. One-year survival of extremely preterm infants after active perinatal care in Sweden. JAMA 301, 2225–2233 (2009).
Baack, M. L., Puumala, S. E., Messier, S. E., Pritchett, D. K. & Harris, W. S. Daily enteral DHA supplementation alleviates deficiency in premature infants. Lipids 51, 423–433 (2016).
Tam, E. W. et al. Early postnatal docosahexaenoic acid levels and improved preterm brain development. Pediatr. Res. 79, 723–730 (2016).
Klevebro, S., Juul, S. E. & Wood, T. R. A more comprehensive approach to the neuroprotective potential of long-chain polyunsaturated fatty acids in preterm infants is needed-should we consider maternal diet and the n-6:n-3 fatty acid ratio? Front. Pediatr. 7, 533 (2019).
Koletzko, B. et al. Should formula for infants provide arachidonic acid along with DHA? A position paper of the European Academy of Paediatrics and the Child Health Foundation. Am. J. Clin. Nutr. 111, 10–16 (2020).
Colombo, J. et al. Long-term effects of LCPUFA supplementation on childhood cognitive outcomes. Am. J. Clin. Nutr. 98, 403–412 (2013).
Colombo, J. et al. acid (DHA) and arachidonic acid (ARA) balance in developmental outcomes. Prostaglandins Leukot. Essent. Fatty Acids 121, 52–56 (2017).
Moon, K., Rao, S. C., Schulzke, S. M., Patole, S. K. & Simmer, K. Longchain polyunsaturated fatty acid supplementation in preterm infants. Cochrane Database Syst. Rev. 12, CD000375 (2016).
Gould, J. F., Roberts, R. M. & Makrides, M. The influence of omega-3 long-chain polyunsaturated fatty acid, docosahexaenoic acid, on child behavioral functioning: a review of randomized controlled trials of DHA supplementation in pregnancy, the neonatal period and infancy. Nutrients. 13, 415 (2021).
Gawlik, N. R., Anderson, A. J., Makrides, M., Kettler, L. & Gould, J. F. The influence of DHA on language development: a review of randomized controlled trials of DHA supplementation in pregnancy, the neonatal period, and infancy. Nutrients 12, 3106 (2020).
Hortensius, L. M., van Elburg, R. M., Nijboer, C. H., Benders, M. & de Theije, C. G. M. Postnatal nutrition to improve brain development in the preterm infant: a systematic review from bench to bedside. Front. Physiol. 10, 961 (2019).
Almaas, A. N. et al. Long-chain polyunsaturated fatty acids and cognition in VLBW infants at 8 years: an RCT. Pediatrics 135, 972–980 (2015).
Almaas, A. N. et al. Diffusion tensor imaging and behavior in premature infants at 8 years of age, a randomized controlled trial with long-chain polyunsaturated fatty acids. Early Hum. Dev. 95, 41–46 (2016).
Alshweki, A. et al. Effects of different arachidonic acid supplementation on psychomotor development in very preterm infants; a randomized controlled trial. Nutr. J. 14, 101 (2015).
Hewawasam, E. et al. DHA supplementation in infants born preterm and the effect on attention at 18 months’ corrected age: follow-up of a subset of the N3RO randomised controlled trial. Br. J. Nutr. 125, 420–431 (2021).
Thanhaeuser, M. et al. A randomized trial of parenteral nutrition using a mixed lipid emulsion containing fish oil in infants of extremely low birth weight: neurodevelopmental outcome at 12 and 24 months corrected age, a secondary outcome analysis. J. Pediatr. 226, 142–148 (2020).
Acknowledgements
We would like to thank all infants and their parents who participated and all clinical and research staff involved in the clinical trial. This study was supported by the Swedish Medical Research Council #2016-01131, The Gothenburg Medical Society, and Government grants under the ALF agreement ALFGBG-717971, De Blindas Vänner and Knut and Alice Wallenberg Clinical Scholars. This work used computing resources of the UK MEDical BIOinformatics partnership—aggregation, integration, visualization, and analysis of large, complex data (UK MED-BIO), which is supported by the Medical Research Council [grant number MR/L01632X/1]. This work used computing resources provided by the Swedish National Infrastructure for Computing (SNIC) at Chalmers Centre for Computational Science and Engineering (C3SE), partially funded by the Swedish Research Council through grant agreement no. 2018-05973. L.M.H. received funding from the Athena grant, “Utrecht Center for Food and Health—research program specialized nutrition,” subsidy from the Dutch Ministry of Economic Affairs, Utrecht Province and the municipality of Utrecht. I.B.-B. received grants from the Sahlgrenska University Hospital (SU 2018-03591, SU 2018-04164, ALFGBG-925851) and the University of Gothenburg (E2018/478).
Author information
Authors and Affiliations
Contributions
All authors have met the Pediatric Research authorship requirements: substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data: L.M.H., W.H., R.A.H., F.G., M.X.A., A.K.N., M.L.T., R.M.v.E., A.H., M.J.N.L.B. Drafting the article or revising it critically for important intellectual content: L.M.H., W.H., K.S., R.A.H., I.M.B.-B., F.G., M.X.A., A.K.N., M.L.T., R.M.v.E., A.H., and M.J.N.L.B. Final approval of the version to be published: L.M.H., W.H., K.S., R.A.H., I.M.B.-B., F.G., M.X.A., A.K.N., M.L.T., R.M.v.E., A.H., and M.J.N.L.B.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent statement
Informed consent from parents or guardians was obtained in writing before the inclusion of the infant in the trial.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Hortensius, L.M., Hellström, W., Sävman, K. et al. Serum docosahexaenoic acid levels are associated with brain volumes in extremely preterm born infants. Pediatr Res 90, 1177–1185 (2021). https://doi.org/10.1038/s41390-021-01645-w
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-021-01645-w
This article is cited by
-
Early parenteral lipid intake supports cerebellar neurometabolism at term-age in preterm infants
Journal of Perinatology (2025)
-
Brain development using a multicomponent intravenous lipid emulsion in preterm infants
BMC Pediatrics (2024)
-
Maternal milk in the NICU: An everyday intervention to improve brain development
Pediatric Research (2024)
-
The influence of nutrition on white matter development in preterm infants: a scoping review
Pediatric Research (2023)
-
Larger brain volumes at term-equivalent age in infants born preterm: an alternative explanation
Pediatric Research (2021)