Abstract
Background
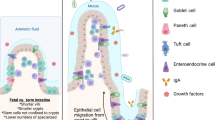
Stem cell therapy has been proven to rescue intestinal injury and stimulate intestinal regeneration in necrotizing enterocolitis (NEC). Specifically, stem cells derived from amniotic fluid (AFSCs) and mesenchymal stem cells (MSCs) derived from bone marrow have shown promising results in the treatment of experimental NEC. This study aims to examine the effects of AFSCs and MSCs on the prevention of intestinal injury during experimental NEC.
Methods
Supernatants from AFSC and MSC cultures were collected to perform proteomic analysis. Prior to NEC induction, mice received intraperitoneal injections of phosphate-buffered saline (PBS), 2 × 106 AFSCs, or 2 × 106 MSCs.
Results
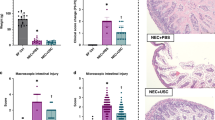
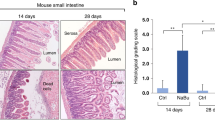
We found that AFSCs grew faster than MSCs. Proteomic analysis indicated that AFSCs are primarily involved in cell development and growth, while MSCs are involved in immune regulation. Administering AFSCs before NEC induction decreased NEC severity and mucosal inflammation. Intestinal proliferation and endogenous stem cell activation were increased after AFSC administration. However, administering MSCs before NEC induction had no beneficial effects.
Conclusions
This study demonstrated that AFSCs and MSCs have different protein release profiles. AFSCs can potentially be used as a preventative strategy for neonates at risk of NEC, while MSCs cannot be used.
Impact
-
AFSCs and MSCs have distinct protein secretory profiles, and AFSCs are primarily involved in cell development and growth, while MSCs are involved in immune regulation.
-
AFSCs are unique in transiently enhancing healthy intestinal epithelial cell growth, which offers protection against the development of experimental NEC.
-
The prevention of NEC via the administration of AFSCs should be evaluated in infants at great risk of developing NEC or in infants with early signs of NEC.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Neu, J. & Walker, W. A. Necrotizing enterocolitis. N. Engl. J. Med. 364, 255–264 (2011).
Yee, W. H. et al. Incidence and timing of presentation of necrotizing enterocolitis in preterm infants. Pediatrics 129, e298–e304 (2012).
Pierro, A. The surgical management of necrotising enterocolitis. Early Hum. Dev. 81, 79–85 (2005).
Thyoka, M. et al. Advanced necrotizing enterocolitis part 1: mortality. Eur. J. Pediatr. Surg. 22, 8–12 (2012).
Tayman, C. et al. Mesenchymal stem cell therapy in necrotizing enterocolitis: a rat study. Pediatr. Res. 70, 489–494 (2011).
Yang, J. et al. Heparin-binding epidermal growth factor-like growth factor and mesenchymal stem cells act synergistically to prevent experimental necrotizing enterocolitis. J. Am. Coll. Surg. 215, 534–545 (2012).
Wei, J., Zhou, Y. & Besner, G. E. Heparin-binding EGF-like growth factor and enteric neural stem cell transplantation in the prevention of experimental necrotizing enterocolitis in mice. Pediatr. Res. 78, 29–37 (2015).
Zani, A. et al. Amniotic fluid stem cells prevent development of ascites in a neonatal rat model of necrotizing enterocolitis. Eur. J. Pediatr. Surg. 24, 57–60 (2014).
Zani, A. et al. Amniotic fluid stem cells improve survival and enhance repair of damaged intestine in necrotising enterocolitis via a COX-2 dependent mechanism. Gut 63, 300–309 (2014).
De Coppi, P. et al. Isolation of amniotic stem cell lines with potential for therapy. Nat. Biotechnol. 25, 100–106 (2007).
Arnhold, S. et al. Amniotic-fluid stem cells: growth dynamics and differentiation potential after a CD-117-based selection procedure. Stem Cells Int. 2011, 715341 (2011).
Li, B. et al. Activation of Wnt signaling by amniotic fluid stem cell-derived extracellular vesicles attenuates intestinal injury in experimental necrotizing enterocolitis. Cell Death Dis. 11, 750 (2020).
Koike, Y. et al. Remote ischemic conditioning counteracts the intestinal damage of necrotizing enterocolitis by improving intestinal microcirculation. Nat. Commun. 11, 4950 (2020).
Walsh, M. C. & Kliegman, R. M. Necrotizing enterocolitis: treatment based on staging criteria. Pediatr. Clin. N. Am. 33, 179–201 (1986).
Bell, M. J. et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann. Surg. 187, 1–7 (1978).
McCulloh, C. J. et al. Evaluating the efficacy of different types of stem cells in preserving gut barrier function in necrotizing enterocolitis. J. Surg. Res. 214, 278–285 (2017).
Antounians, L. et al. The regenerative potential of amniotic fluid stem cell extracellular vesicles: lessons learned by comparing different isolation techniques. Sci. Rep. 9, 1837 (2019).
Zani, A. et al. A spectrum of intestinal injury models in neonatal mice. Pediatr. Surg. Int. 32, 65–70 (2016).
Dvorak, B. et al. Epidermal growth factor reduces the development of necrotizing enterocolitis in a neonatal rat model. Am. J. Physiol. Gastrointest. Liver Physiol. 282, G156–G164 (2002).
Ran-Ressler, R. R. et al. Branched chain fatty acids reduce the incidence of necrotizing enterocolitis and alter gastrointestinal microbial ecology in a neonatal rat model. PLoS ONE 6, e29032 (2011).
Li, B. et al. Impaired Wnt/beta-catenin pathway leads to dysfunction of intestinal regeneration during necrotizing enterocolitis. Cell Death Dis. 10, 743 (2019).
Li, B. et al. Inhibition of corticotropin-releasing hormone receptor 1 and activation of receptor 2 protect against colonic injury and promote epithelium repair. Sci. Rep. 7, 46616 (2017).
Pozzobon, M., Piccoli, M., Schiavo, A. A., Atala, A. & De Coppi, P. Isolation of c-Kit+ human amniotic fluid stem cells from second trimester. Methods Mol. Biol. 1035, 191–198 (2013).
Schiavo, A. A. et al. Endothelial properties of third-trimester amniotic fluid stem cells cultured in hypoxia. Stem Cell Res. Ther. 6, 209 (2015).
Koike, Y. et al. The intestinal injury caused by ischemia-reperfusion is attenuated by amniotic fluid stem cells via the release of tumor necrosis factor-stimulated gene 6 protein. FASEB J. 34, 6824–6836 (2020).
Ceulemans, H. & Bollen, M. Functional diversity of protein phosphatase-1, a cellular economizer and reset button. Physiological Rev. 84, 1–39 (2004).
Firth, S. M. & Baxter, R. C. Cellular actions of the insulin-like growth factor binding proteins. Endocr. Rev. 23, 824–854 (2002).
Ferry, R. J. Jr., Katz, L. E., Grimberg, A., Cohen, P. & Weinzimer, S. A. Cellular actions of insulin-like growth factor binding proteins. Horm. Metab. Res. 31, 192–202 (1999).
Grimberg, A. & Cohen, P. Role of insulin-like growth factors and their binding proteins in growth control and carcinogenesis. J. Cell. Physiol. 183, 1–9 (2000).
Jogie-Brahim, S., Feldman, D. & Oh, Y. Unraveling insulin-like growth factor binding protein-3 actions in human disease. Endocr. Rev. 30, 417–437 (2009).
Granata, R. et al. Dual effects of IGFBP-3 on endothelial cell apoptosis and survival: involvement of the sphingolipid signaling pathways. FASEB J. 18, 1456–1458 (2004).
Chang, K. H. et al. IGF binding protein-3 regulates hematopoietic stem cell and endothelial precursor cell function during vascular development. Proc. Natl Acad. Sci. USA 104, 10595–10600 (2007).
Takaoka, M. et al. Epidermal growth factor receptor regulates aberrant expression of insulin-like growth factor-binding protein 3. Cancer Res. 64, 7711–7723 (2004).
Good, M. et al. Breast milk protects against the development of necrotizing enterocolitis through inhibition of Toll-like receptor 4 in the intestinal epithelium via activation of the epidermal growth factor receptor. Mucosal Immunol. 8, 1166–1179 (2015).
Nousbeck, J. et al. Insulin-like growth factor-binding protein 7 regulates keratinocyte proliferation, differentiation and apoptosis. J. Investig. Dermatol. 130, 378–387 (2010).
Skardal, A. et al. Bioprinted amniotic fluid-derived stem cells accelerate healing of large skin wounds. Stem Cells Transl. Med. 1, 792–802 (2012).
Sato, Y. et al. Prophylactic therapy with human amniotic fluid stem cells improved survival in a rat model of lipopolysaccharide-induced neonatal sepsis through immunomodulation via aggregates with peritoneal macrophages. Stem Cell Res. Ther. 11, 300 (2020).
Thomas, H. Intestinal homeostasis is reliant on self-maintaining macrophages. Nat. Rev. Gastroenterol. Hepatol. 15, 656–657 (2018).
McCulloh, C. J., Olson, J. K., Zhou, Y., Wang, Y. & Besner, G. E. Stem cells and necrotizing enterocolitis: a direct comparison of the efficacy of multiple types of stem cells. J. Pediatr. Surg. 52, 999–1005 (2017).
Dvorakova, J., Hruba, A., Velebny, V. & Kubala, L. Isolation and characterization of mesenchymal stem cell population entrapped in bone marrow collection sets. Cell Biol. Int. 32, 1116–1125 (2008).
Zhu, H. et al. A protocol for isolation and culture of mesenchymal stem cells from mouse compact bone. Nat. Protoc. 5, 550–560 (2010).
Acknowledgements
Proteomic analyses were performed by the Center for Advanced Proteomics Analyses, a Node of the Canadian Genomic Innovation Network that is supported by the Canadian Government through Genome Canada. B.L. is the recipient of a Restracomp Fellowship, The Hospital for Sick Children, and an Early Career Award Program grant from the Thrasher Research Fund (14503). A.P. is the recipient of a Canadian Institutes of Health Research (CIHR) Foundation Grant (353857) and was also supported by the Robert M. Filler Chair of Surgery, The Hospital for Sick Children.
Author information
Authors and Affiliations
Contributions
B.L., C.L., M.C., J.S.O’C., S.R.B., H.M., M.A., N.G., K.C.J.-H., and P.M.S.: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing, and final approval of the manuscript. P.M. and A.P.: conception and design, financial support, and final approval of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Patient consent
Patient consent was not required for this experimental study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Li, B., Lee, C., Cadete, M. et al. Amniotic fluid stem cell administration can prevent epithelial injury from necrotizing enterocolitis. Pediatr Res 91, 101–106 (2022). https://doi.org/10.1038/s41390-021-01657-6
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-021-01657-6
This article is cited by
-
Amniotic fluid: its role in fetal development and beyond
Journal of Perinatology (2025)
-
Characteristics, progression, management, and outcomes of NEC: a retrospective cohort study
Pediatric Surgery International (2024)
-
Necrotizing enterocolitis: current understanding of the prevention and management
Pediatric Surgery International (2024)
-
Translating regenerative medicine therapies in neonatal necrotizing enterocolitis
Pediatric Research (2024)
-
Stem cell therapy as a promising strategy in necrotizing enterocolitis
Molecular Medicine (2022)