Abstract
Background
Early-life metabolic derangements in HIV-exposed uninfected (HEU) infants have been reported.
Methods
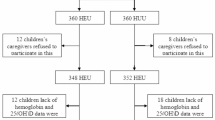
Pregnant women with HIV and HIV-uninfected pregnant women were enrolled with their newborns in a US cohort from 2011 to 2015. We measured cord insulin, C-peptide, and metabolic cytokines of HEU and HIV-unexposed uninfected (HUU) newborns using ELISA and metabolites, lipid subspecies, and eicosanoids via liquid chromatography/mass spectrometry. Linear regression was employed to assess the association of intrauterine HIV/ART with insulin and C-peptide. Graphical lasso regression was used to identify differences between metabolite/lipid subspecies networks associated with C-peptide.
Results
Of 118 infants, 56 were HEU, ART exposed. In adjusted analyses, mean cord insulin (β = 0.295, p = 0.03) and C-peptide (β = 0.522, p < 0.01) were significantly higher in HEU vs. HUU newborns. HEU neonates exhibited primarily positive associations between complex lipids and C-peptide, indicative of fuel storage, and augmented associations between cord eicosanoids and cytokines. HUU neonates exhibited negative associations with lipids and C-peptide indicative of increased fuel utilization.
Conclusion
Higher cord insulin and C-peptide in HEU vs. HUU newborns as well as differences in cord metabolites, metabolic-related cytokines, and eicosanoids may reflect a propensity for fuel storage and an inflammatory milieu suggestive of fetal metabolic changes associated with in utero HIV/ART exposure.
Impact
-
There is a paucity of studies assessing cord blood and neonatal metabolic health in HIV-exposed uninfected (HEU) newborns, an increasing population worldwide.
-
Compared to HIV-unexposed uninfected (HUU) newborns, HEU newborns exhibit alterations in fuel homeostasis and an inflammatory milieu associated with in utero HIV/antiretroviral therapy (ART) exposure.
-
The long-term implications of these neonatal findings are as yet unknown, but merit continued evaluation as this important and growing population ages into adulthood.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Barker, D. J. Rise and fall of Western diseases. Nature 338, 371–372 (1989).
Slogrove, A. L., Powis, K. M., Johnson, L. F., Stover, J. & Mahy, M. Estimates of the global population of children who are HIV-exposed and uninfected, 2000-18: a modelling study. Lancet Glob. Health 8, e67–e75 (2020).
Brown, T. T. et al. Antiretroviral therapy and the prevalence and incidence of diabetes mellitus in the multicenter AIDS cohort study. Arch. Intern. Med. 165, 1179–1184 (2005).
Nix, L. M. & Tien, P. C. Metabolic syndrome, diabetes, and cardiovascular risk in HIV. Curr. HIV/AIDS Rep. 11, 271–278 (2014).
Grant, P. M. et al. Long-term body composition changes in antiretroviral-treated Hiv-infected individuals. AIDS 30, 2805–2813 (2016).
Jao, J. & Abrams, E. J. Metabolic complications of in utero maternal HIV and antiretroviral exposure in HIV-exposed infants. Pediatr. Infect. Dis. J. 33, 734–740 (2014).
Jao, J. et al. Lower preprandial insulin and altered fuel use in HIV/antiretroviral-exposed infants in Cameroon. J. Clin. Endocrinol. Metab. 100, 3260–3269 (2015).
Jao, J. et al. Lower mitochondrial DNA and altered mitochondrial fuel metabolism in HIV-exposed uninfected infants in Cameroon. AIDS 31, 2475–2481 (2017).
Chen, J. Y. et al. Highly active antiretroviral therapy and adverse birth outcomes among HIV-infected women in Botswana. J. Infect. Dis. 206, 1695–1705 (2012).
Cote, H. C. et al. Perinatal exposure to antiretroviral therapy is associated with increased blood mitochondrial DNA levels and decreased mitochondrial gene expression in infants. J. Infect. Dis. 198, 851–859 (2008).
Schoeman, J. C. et al. Fetal metabolic stress disrupts immune homeostasis and induces proinflammatory responses in human immunodeficiency virus type 1- and combination antiretroviral therapy-exposed infants. J. Infect. Dis. 216, 436–446 (2017).
Regnault, N. et al. Higher cord C-peptide concentrations are associated with slower growth rate in the 1st year of life in girls but not in boys. Diabetes 60, 2152–2159 (2011).
Carlsen, E. M. et al. The association between newborn regional body composition and cord blood concentrations of C-peptide and insulin-like growth factor I. PLoS ONE 10, e0121350 (2015).
Zhang, D. L. et al. Cord blood insulin, IGF-I, IGF-II, leptin, adiponectin and ghrelin, and their associations with insulin sensitivity, beta-cell function and adiposity in infancy. Diabet. Med. 35, 1412–1419 (2018).
Carpenter, M. W. & Coustan, D. R. Criteria for screening tests for gestational diabetes. Am. J. Obstet. Gynecol. 144, 768–773 (1982).
Olsen, I. E., Groveman, S. A., Lawson, M. L., Clark, R. H. & Zemel, B. S. New intrauterine growth curves based on United States data. Pediatrics 125, e214–e224 (2010).
Lowe, W. L. Jr. et al. Hyperglycemia and Adverse Pregnancy Outcome Follow-up Study (Hapo Fus): Maternal Gestational Diabetes Mellitus and Childhood Glucose Metabolism. Diabetes Care 42, 372–380 (2019).
Bylesjö, M. et al. Opls discriminant analysis: combining the strengths of PLS-DA and Simca classification. J. Chemometrics 20, 341–351 (2006).
Friedman, J., Hastie, T. & Tibshirani, R. Sparse inverse covariance estimation with the Graphical Lasso. Biostatistics 9, 432–441 (2008).
Cohen, J. A power primer. Psychol. Bull. 112, 155–159 (1992).
Jones, A. G. & Hattersley, A. T. The clinical utility of C-peptide measurement in the care of patients with diabetes. Diabet. Med. 30, 803–817 (2013).
El Beitune, P. et al. Effect of maternal use of antiretroviral agents on serum insulin levels of the newborn infant. Diabetes Care 28, 856–859 (2005).
Sridhar, S. B., Ferrara, A., Ehrlich, S. F., Brown, S. D. & Hedderson, M. M. Risk of large-for-gestational-age newborns in women with gestational diabetes by race and ethnicity and body mass index categories. Obstet. Gynecol. 121, 1255–1262 (2013).
Spranger, J. et al. Inflammatory cytokines and the risk to develop Type 2 diabetes: results of the prospective population-based European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Diabetes 52, 812–817 (2003).
Liu, S. et al. A prospective study of inflammatory cytokines and diabetes mellitus in a multiethnic cohort of postmenopausal women. Arch. Intern. Med. 167, 1676–1685 (2007).
Pradhan, A. D., Manson, J. E., Rifai, N., Buring, J. E. & Ridker, P. M. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA 286, 327–334 (2001).
Vinitha, R. et al. Adiponectin, leptin, interleukin−6 and Hba1c in the prediction of incident type 2 diabetes: a nested case-control study in Asian Indian men with impaired glucose tolerance. Diabetes Res. Clin. Pract. 109, 340–346 (2015).
Betene, A. D. C. et al. Interleukin−6, high sensitivity C-reactive protein, and the development of Type 2 diabetes among HIV-positive patients taking antiretroviral therapy. J. Acquir. Immune Defic. Syndr. 67, 538–546 (2014).
Kamimura, D., Ishihara, K. & Hirano, T. Il-6 signal transduction and its physiological roles: the signal orchestration model. Rev. Physiol. Biochem. Pharmacol. 149, 1–38 (2003).
Jones, S. A., Horiuchi, S., Topley, N., Yamamoto, N. & Fuller, G. M. The soluble interleukin 6 receptor: mechanisms of production and implications in disease. FASEB J. 15, 43–58 (2001).
Kristiansen, O. P. & Mandrup-Poulsen, T. Interleukin-6 and diabetes: the good, the bad, or the indifferent? Diabetes 54, S114–S124 (2005).
Ng, P. C. et al. Plasma Ghrelin and Resistin concentrations are suppressed in infants of insulin-dependent diabetic mothers. J. Clin. Endocrinol. Metab. 89, 5563–5568 (2004).
Mohamed, M. H. et al. Cord blood Resistin and Adiponectin in term newborns of diabetic mothers. Arch. Med. Sci. 6, 558–566 (2010).
Silswal, N. et al. Human Resistin stimulates the pro-inflammatory cytokines Tnf-Alpha and Il-12 in macrophages by Nf-Kappab-dependent pathway. Biochem. Biophys. Res. Commun. 334, 1092–1101 (2005).
Kim, K. H., Lee, K., Moon, Y. S. & Sul, H. S. A cysteine-rich adipose tissue-specific secretory factor inhibits adipocyte differentiation. J. Biol. Chem. 276, 11252–11256 (2001).
Catalano, P. M., Presley, L., Minium, J. & Hauguel-de Mouzon, S. Fetuses of obese mothers develop insulin resistance in utero. Diabetes Care 32, 1076–1080 (2009).
Addy, C. L. et al. Hypoadiponectinemia is associated with insulin resistance, hypertriglyceridemia, and fat redistribution in human immunodeficiency virus-infected patients treated with highly active antiretroviral therapy. J. Clin. Endocrinol. Metab. 88, 627–636 (2003).
Duncan, B. B. et al. Adiponectin and the development of Type 2 diabetes: The Atherosclerosis Risk in Communities Study. Diabetes. 53, 2473–2478 (2004).
Luo, Z. C. et al. Maternal and fetal leptin, adiponectin levels and associations with fetal insulin sensitivity. Obesity 21, 210–216 (2013).
Basu, S. et al. In utero gender dimorphism of adiponectin reflects insulin sensitivity and adiposity of the fetus. Obesity 17, 1144–1149 (2009).
Pinar, H. et al. High molecular mass multimer complexes and vascular expression contribute to high adiponectin in the fetus. J. Clin. Endocrinol. Metab. 93, 2885–2890 (2008).
Weyermann, M., Beermann, C., Brenner, H. & Rothenbacher, D. Adiponectin and leptin in maternal serum, cord blood, and breast milk. Clin. Chem. 52, 2095–2102 (2006).
McCormack, S. E. et al. Circulating branched-chain amino acid concentrations are associated with obesity and future insulin resistance in children and adolescents. Pediatr. Obes. 8, 52–61 (2013).
Newgard, C. B. et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 9, 311–326 (2009).
Davis, T. A., Burrin, D. G., Fiorotto, M. L., Reeds, P. J. & Jahoor, F. Roles of insulin and amino acids in the regulation of protein synthesis in the neonate. J. Nutr. 128, 347S–350S (1998).
Acknowledgements
We would like to thank all the study participants and staff at the Mount Sinai Medical Center Obstetrical Clinic. This work was supported by K23HD070760 and by the Icahn School of Medicine at Mount Sinai Dean’s Office and ConduITS—the Institutes for Translational Sciences (CTSA) (UL1TR001433). Y.Q. was supported by NIDDK P60DK020541. I.J.K. was supported by grants NIDDK P60DK020541 (Einstein DRTC) and NIAID 1U19AI091175 (Einstein CMCR), and had support of a S10 SIG Award for the Sciex 6500+ QTRAP (1S10OD021798-01) which was used to perform the mass spectrometric assay evaluations in this study.
Funding
This work was supported in part by NICHD K23HD070760 and by the Icahn School of Medicine at Mount Sinai Dean’s Office and ConduITS—the Institutes for Translational Sciences (CTSA) (UL1TR001433). Y.Q. is supported by NIDDK P60DK020541. I.J.K. was supported by grants NIDDK P60DK020541 (Einstein DRTC) and NIAID 1U19AI091175 (Einstein CMCR), and the mass spectrometric work at Einstein DRTC-SIMC was supported by a S10 SIG Award for the Sciex 6500+ QTRAP (1S10OD021798-01).
Author information
Authors and Affiliations
Contributions
J.J., R.S.S., E.J.A. and D.L. conceptualized this study. J.J. wrote the first draft of the manuscript, and I.J.K. assisted with important edits. J.J. conducted data analyses involving clinical data, while L.C.B. conducted the data analyses involving omics data, and S.S. assisted with all data analyses. L.C.B. contributed to the “Methods” section. I.J.K. contributed to the “Results” and “Discussion” section. J.J., I.J.K., M.E.G. and D.L. made significant edits before completing the final manuscript. Y.Q., T.A.K., B.K., S.A., L.M., E.J.A. and R.S.S. all reviewed and edited the manuscript. J.J. finalized the manuscript. All authors approved the final manuscript.
Corresponding author
Ethics declarations
Consent statement
All participants provided written informed consent. This study was approved by the Institutional Review Board of the Icahn School of Medicine at Mount Sinai.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Jao, J., Balmert, L.C., Sun, S. et al. Distinct cord blood C-peptide, adipokine, and lipidomic signatures by in utero HIV exposure. Pediatr Res 92, 233–241 (2022). https://doi.org/10.1038/s41390-021-01705-1
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41390-021-01705-1