Abstract
Background
Preterm infants frequently experience intermittent hypoxia (IH) episodes, rendering them susceptible to oxidative stress and gut dysbiosis. We tested the hypothesis that early supplementation with antioxidants and/or fish oil promotes gut biodiversity and mitigates IH-induced gut injury.
Methods
Newborn rats were exposed to neonatal IH from birth (P0) to P14 during which they received daily oral supplementation with: (1) coenzyme Q10 (CoQ10) in olive oil, (2) fish oil, (3) glutathione nanoparticles (nGSH), (4) CoQ10 + fish oil, or (5) olive oil (placebo control). Pups were placed in room air (RA) from P14 to P21 with no further treatment. RA controls were similarly treated. Stool samples were assessed for microbiota and terminal ileum for histopathology and morphometry, total antioxidant capacity, lipid peroxidation, and biomarkers of gut injury.
Results
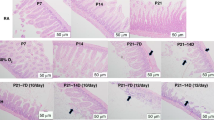
Neonatal IH induced histopathologic changes consistent with necrotizing enterocolitis, which were associated with increased lipid peroxidation, toll-like receptor, transforming growth factor, and nuclear factor kappa B. Combination of CoQ10 + fish oil and nGSH were most effective for preserving gut integrity, reducing biomarkers of gut injury, and increasing commensal organisms.
Conclusions
Combination of antioxidants and fish oil may confer synergistic benefits to mitigate IH-induced injury in the terminal ileum.
Impact
-
Antioxidant and fish oil (PUFA) co-treatment was most beneficial for reducing neonatal IH-induced gut injury.
-
The synergistic effects of antioxidant and fish oil is likely due to prevention of IH-induced ROS attack on lipids, thus preserving and augmenting its therapeutic benefits.
-
Combination treatment was also effective for increasing the abundance of the non-pathogenic Firmicutes phylum, which is associated with a healthy gastrointestinal system of the newborn.
-
Extremely low gestational age neonates who are at high risk for frequent, repetitive neonatal IH and oxidative stress-induced diseases may benefit from this combination therapy.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Saugstad, O. D. The oxygen radical disease in neonatology. Indian. J. Pediatr. 56, 585–593 (1989).
Saugstad, O. D. Oxygen toxicity in the neonatal period. Acta Paediatr. Scand. 79, 881–892 (1990).
Saugstad, O. D. Update on oxygen radical disease in neonatology. Curr. Opin. Obstet. Gynecol. 13, 147–153 (2001).
Di Fiore, J. M., Martin, R. J. & Gauda, E. B. Apnea of prematurity-perfect storm. Respir. Physiol. Neurobiol. 189, 213–222 (2013).
Martin, R. J., Di Fiore, J. M., Macfarlane, P. M. & Wilson, C. G. Physiologic basis for intermittent hypoxic episodes in preterm infants. Adv. Exp. Med. Biol. 758, 351–358 (2012).
Coleman, R. J. et al. Effects of brief, clustered versus dispersed hypoxic episodes on systemic and ocular growth factors in a rat model of oxygen-induced retinopathy. Pediatr. Res. 64, 50–55 (2008).
Di Fiore, J. M. et al. The relationship between patterns of intermittent hypoxia and retinopathy of prematurity in preterm infants. Pediatr. Res. 72, 606–612 (2012).
Kim, M., Christley, S., Alverdy, J. C., Liu, D. & An, G. Immature oxidative stress management as a unifying principle in the pathogenesis of necrotizing enterocolitis: insights from an agent-based model. Surg. Infect. 13, 18–32 (2012).
Baregamian, N. et al. Intestinal mitochondrial apoptotic signaling is activated during oxidative stress. Pediatr. Surg. Int. 27, 871–877 (2011).
Baregamian, N. et al. Tumor necrosis factor-alpha and apoptosis signal-regulating kinase 1 control reactive oxygen species release, mitochondrial autophagy, and c-Jun N-terminal kinase/p38 phosphorylation during necrotizing enterocolitis. Oxid. Med. Cell. Longev. 2, 297–306 (2009).
Hirano, K. et al. Blood transfusion increases radical promoting non-transferrin bound iron in preterm infants. Arch. Dis. Child. Fetal Neonatal Ed. 84, F188–F193 (2001).
Kehrer, J. P. The Haber-Weiss reaction and mechanisms of toxicity. Toxicology 149, 43–50 (2000).
Cheng, Z. & Li, Y. What is responsible for the initiating chemistry of iron-mediated lipid peroxidation: an update. Chem. Rev. 107, 748–766 (2007).
Cao, J. Y. & Dixon, S. J. Mechanisms of ferroptosis. Cell. Mol. Life Sci. 73, 2195–2209 (2016).
Miess, H. et al. The glutathione redox system is essential to prevent ferroptosis caused by impaired lipid metabolism in clear cell renal cell carcinoma. Oncogene 37, 5435–5450 (2018).
Sinha, R. et al. Oral supplementation with liposomal glutathione elevates body stores of glutathione and markers of immune function. Eur. J. Clin. Nutr. 72, 105–111 (2018).
Nagapan, T. S., Lim, W. N., Basri, D. F. & Ghazali, A. R. Oral supplementation of L-glutathione prevents ultraviolet B-induced melanogenesis and oxidative stress in BALB/c mice. Exp. Anim. 68, 541–548 (2019).
Gould, R. L. & Pazdro, R. Impact of supplementary amino acids, micronutrients, and overall diet on glutathione homeostasis. Nutrients 11, 1056 (2019).
Sangsefidi, Z. S., Yaghoub, I. F., Hajiahmadi, S. & Hosseinzadeh, M. The effect of coenzyme Q10 supplementation on oxidative stress: a systematic review and meta-analysis of randomized controlled clinical trials. Food Sci. Nutr. 8, 1766–1776 (2020).
Bersuker, K. et al. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature 575, 688–692 (2019).
Bruggeman, B. K. et al. The absorptive effects of orobuccal non-liposomal nano-sized glutathione on blood glutathione parameters in healthy individuals: a pilot study. PLoS ONE 14, e0215815 (2019).
Miles, E. A., Childs, C. E. & Calder, P. C. Long-chain polyunsaturated fatty acids (LCPUFAs) and the developing immune system: a narrative review. Nutrients 13, 247 (2021).
Younge, N., Yang, Q. & Seed, P. C. Enteral high fat-polyunsaturated fatty acid blend alters the pathogen composition of the intestinal microbiome in premature infants with an enterostomy. J. Pediatr. 181, 93–101 (2017).
Skouroliakou, M. et al. A double-blind, randomized clinical trial of the effect of omega-3 fatty acids on the oxidative stress of preterm neonates fed through parenteral nutrition. Eur. J. Clin. Nutr. 64, 940–947 (2010).
Valentine, G., Chu, D. M., Stewart, C. J. & Aagaard, K. M. Relationships between perinatal interventions, maternal-infant microbiomes, and neonatal outcomes. Clin. Perinatol. 45, 339–355 (2018).
Lee, J. K., Hern Tan, L. T., Ramadas, A., Ab Mutalib, N. S. & Lee, L. H. Exploring the role of gut bacteria in health and disease in preterm neonates. Int. J. Environ. Res. Public Health 17, 6963 (2020).
Olm, M. R. et al. Necrotizing enterocolitis is preceded by increased gut bacterial replication, Klebsiella, and fimbriae-encoding bacteria. Sci. Adv. 5, 5727 (2019).
Cuna, A., Morowitz, M. J., Ahmed, I., Umar, S. & Sampath, V. Dynamics of the preterm gut microbiome in health and disease. Am. J. Physiol. Gastrointest. Liver Physiol. 320, G411–G419 (2021).
Ko, C. Y. et al. Disruption of sleep architecture in Prevotella enterotype of patients with obstructive sleep apnea-hypopnea syndrome. Brain Behav. 9, e01287 (2019).
Ko, C. Y. et al. Gut microbiota in obstructive sleep apnea-hypopnea syndrome: disease-related dysbiosis and metabolic comorbidities. Clin. Sci. 133, 905–917 (2019).
Tripathi, A. et al. Intermittent hypoxia and hypercapnia reproducibly change the gut microbiome and metabolome across rodent model systems. mSystems 4, e00058-19 (2019).
Tian, Y. M. et al. Short-term chronic intermittent hypobaric hypoxia alters gut microbiota composition in rats. Biomed. Environ. Sci. 31, 898–901 (2018).
Moreno-Indias, I. et al. Intermittent hypoxia alters gut microbiota diversity in a mouse model of sleep apnoea. Eur. Respir. J. 45, 1055–1065 (2015).
Ianiro, G. et al. Effect of an extra-virgin olive oil enriched with probiotics or antioxidants on functional dyspepsia: a pilot study. Eur. Rev. Med. Pharmacol. Sci. 17, 2085–2090 (2013).
Brugè, F. et al. Olive oil supplemented with coenzyme Q(10): effect on plasma and lipoprotein oxidative status. Biofactors 38, 249–256 (2012).
Huertas, J. R. et al. Virgin olive oil and coenzyme Q10 protect heart mitochondria from peroxidative damage during aging. Biofactors 9, 337–343 (1999).
Barden, A. E. et al. The effect of n-3 fatty acids and coenzyme Q10 supplementation on neutrophil leukotrienes, mediators of inflammation resolution and myeloperoxidase in chronic kidney disease. Prostaglandins Other Lipid Mediat. 136, 1–8 (2018).
Zhang, Y. P. et al. Diabetic neuropathic pain development in type 2 diabetic mouse model and the prophylactic and therapeutic effects of coenzyme Q10. Neurobiol. Dis. 58, 169–178 (2013).
Beharry, K. D. et al. Co-enzyme Q10 and n-3 polyunsaturated fatty acid supplementation reverse intermittent hypoxia-induced growth restriction and improved antioxidant profiles in neonatal rats. Antioxidants 6, 103 (2017).
Cai, C., Aranda, J. V., Valencia, G. B., Xu, J. & Beharry, K. D. Chronic intermittent hypoxia causes lipid peroxidation and altered phase 1 drug metabolizing enzymes in the neonatal rat liver. React. Oxyg. Species 3, 218–236 (2017).
Martin, R. J., Wang, K., Köroğlu, O., Di Fiore, J. & Kc, P. Intermittent hypoxic episodes in preterm infants: do they matter? Neonatology 100, 303–310 (2011).
Leger, T. et al. Dietary canolol protects the heart against the deleterious effects induced by the association of rapeseed oil, vitamin E and coenzyme Q10 in the context of a high-fat diet. Nutr. Metab. 15, 15 (2018).
Staude, B. et al. The microbiome and preterm birth: a change in paradigm with profound implications for pathophysiologic concepts and novel therapeutic strategies. Biomed. Res. Int. 2018, 7218187 (2018).
Fundora, J. B. et al. Intestinal dysbiosis and necrotizing enterocolitis: assessment for causality using Bradford Hill criteria. Pediatr. Res. 87, 235–248 (2020).
Flemer, B. et al. Fecal microbiota variation across the lifespan of the healthy laboratory rat. Gut Microbes 8, 428–439 (2017).
Costantini, L., Molinari, R., Farinon, B. & Merendino, N. Impact of omega 3 fatty acids on gut microbiota. Int. J. Mol. Sci. 18, 2645 (2017).
Lutgendorff, F. et al. Probiotics prevent intestinal barrier dysfunction in acute pancreatitis in rats via induction of ileal mucosal glutathione synthesis. PLoS ONE 4, e4512 (2009).
Bains, V. K. & Bains, R. The antioxidant master glutathione and periodontal health. Dent. Res. J. 12, 389–405 (2015).
Wu, J. et al. Disrupted intestinal structure in a rat model of intermittent hypoxia. Mol. Med. Rep. 13, 4407–4413 (2016).
Frost, B. L., Jilling, T. & Caplan, M. S. The importance of pro-inflammatory signaling in neonatal necrotizing enterocolitis. Semin. Perinatol. 32, 100–106 (2008).
Tanner, S. M. et al. Pathogenesis of necrotizing enterocolitis: modeling the innate immune response. Am. J. Pathol. 185, 4–16 (2015).
Jilling, T. et al. The roles of bacteria and TLR4 in rat and murine models of necrotizing enterocolitis. J. Immunol. 177, 3273–3282 (2006).
Lu, J., Jilling, T., Li, D. & Caplan, M. S. Polyunsaturated fatty acid supplementation alters proinflammatory gene expression and reduces the incidence of necrotizing enterocolitis in a neonatal rat model. Pediatr. Res. 61, 427–432 (2007).
Bauche, D. & Marie, J. Transforming growth factor β: a master regulator of the gut microbiota and immune cell interactions. Clin. Transl. Immunol. 6, e136 (2017).
Hunter, C. J. & De Plaen, I. G. Inflammatory signaling in NEC: role of NF-κB, cytokines and other inflammatory mediators. Pathophysiology 21, 55–65 (2014).
Jourd’heuil, D., Morise, Z., Conner, E. M., Kurose, I. & Grisham, M. B. Oxidant-regulation of gene expression in the chronically inflamed intestine. Keio J. Med. 46, 10–15 (1997).
Claud, E. C. & Walker, W. A. Hypothesis: inappropriate colonization of the premature intestine can cause neonatal necrotizing enterocolitis. FASEB J. 15, 1398–1403 (2001).
Yin, J. et al. Development of an antioxidant system after early weaning in piglets. J. Anim. Sci. 92, 612–619 (2014).
Funding
This work was made possible through the Eunice Kennedy Shriver National Institute of Child Health & Human Development Grant #U54HD071594 and the Alicia and Madu Rao Research Foundation Grant EIN 262145011.
Author information
Authors and Affiliations
Contributions
All authors have made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; drafting the article; revising it critically for important intellectual content; and final approval of the version to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Bodkin, D., Cai, C.L., Manlapaz-Mann, A. et al. Neonatal intermittent hypoxia, fish oil, and/or antioxidant supplementation on gut microbiota in neonatal rats. Pediatr Res 92, 109–117 (2022). https://doi.org/10.1038/s41390-021-01707-z
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-021-01707-z
This article is cited by
-
Gut microbiome and inflammation in response to increasing intermittent hypoxia in the neonatal rat
Pediatric Research (2025)
-
Gastroprotective and microbiome-modulating effects of ubiquinol in rats with radiation-induced enteropathy
Animal Microbiome (2024)