Abstract
Background
The objective of this study was to systematically review the literature to determine the effect of combined hypothermia (HTH) and mesenchymal stem cell (MSC) therapy (administered during or immediately before or after HTH) compared with HTH alone on brain injury and neurobehavioural outcomes in animal models of neonatal hypoxic–ischaemic encephalopathy.
Methods
Primary outcomes assessed were neuropathological measures and neurobehavioural measures of brain outcome. Secondary outcomes were brain protein proinflammatory cytokine status. Risk of bias (ROB) was assessed with the Systematic Review Centre for Laboratory Animal Experimentation (SYRCLE) ROB assessment tool.
Results
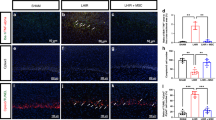
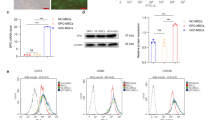
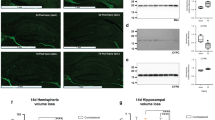
Of 393 studies identified, 3 studies in postnatal day 7 (P7) male Sprague–Dawley rats met the inclusion criteria. Meta-analyses were undertaken for neuropathological measures (apoptotic cells, astrocytes, microglia), neurobehavioral measures (rotarod test and negative geotaxis), and proinflammatory cytokine levels. Two of the three studies scored low or unclear ROB across all measures. Treatment with HTH-MSCs together significantly improved astrocyte optical density by standardised mean difference (SMD) of 0.71 [95% confidence interval (CI) −1.14, −0.28]. No other measures showed significant differences.
Conclusions
There is insufficient preclinical data to confirm the efficacy of combined HTH-MSC therapy over HTH alone. Future studies should utilise a reporting checklist such as in SYRCLE or Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines to improve reporting standards.
Impact
-
Very few articles investigating the use of MSCs for the treatment of hypoxic–ischaemic encephalopathy are clinically relevant.
-
Continuing to publish studies in models of hypoxic–ischaemic encephalopathy without the inclusion of HTH therapy does not progress the field towards improved clinical outcomes.
-
This study shows that HTH and MSC therapy improves measures of astrogliosis.
-
More studies are required to establish the efficacy of HTH and MSCs on measures of neuropathology and neurobehavior.
-
The reporting of preclinical data in this space could be improved by using reporting checklists such as the SYRCLE or ARRIVE tools.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Cotten, C. M. et al. Feasibility of autologous cord blood cells for infants with hypoxic-ischemic encephalopathy. J. Pediatr. 164, 973–979.e971 (2014).
Tsuji, M. et al. Autologous cord blood cell therapy for neonatal hypoxic-ischaemic encephalopathy: a pilot study for feasibility and safety. Sci. Rep. 10, 4603 (2020).
Archambault, J. et al. Therapeutic potential of mesenchymal stromal cells for hypoxic ischemic encephalopathy: a systematic review and meta-analysis of preclinical studies. PLoS ONE 12, e0189895–e0189895 (2017).
Kim, Y. E. et al. Thrombin preconditioning enhances therapeutic efficacy of human Wharton’s jelly-derived mesenchymal stem cells in severe neonatal hypoxic ischemic encephalopathy. Int. J. Mol. Sci. 20, 2477 (2019).
Herz, J. et al. Interaction between hypothermia and delayed mesenchymal stem cell therapy in neonatal hypoxic-ischemic brain injury. Brain Behav. Immun. 70, 118–130 (2018).
Amos, P. J. et al. Human adipose-derived stromal cells accelerate diabetic wound healing: impact of cell formulation and delivery. Tissue Eng. Part A 16, 1595–1606 (2010).
Hemeda, H. et al. Interferon-Γ and tumor necrosis factor-α differentially affect cytokine expression and migration properties of mesenchymal stem cells. Stem Cells Dev. 19, 693–706 (2010).
Lee, K. et al. Effects of serial passage on the characteristics and chondrogenic differentiation of canine umbilical cord matrix derived mesenchymal stem cells. Asian-Australas. J. Anim. Sci. 26, 588 (2013).
Cui, X. et al. Transplantation of mesenchymal stem cells preconditioned with diazoxide, a mitochondrial ATP-sensitive potassium channel opener, promotes repair of myocardial infarction in rats. Tohoku J. Exp. Med. 220, 139–147 (2010).
Pessina, A. et al. Mesenchymal stromal cells primed with paclitaxel attract and kill leukaemia cells, inhibit angiogenesis and improve survival of leukaemia-bearing mice. Br. J. Haematol. 160, 766–778 (2013).
Kang, H. et al. The therapeutic effects of human mesenchymal stem cells primed with sphingosine-1 phosphate on pulmonary artery hypertension. Stem Cells Dev. 24, 1658–1671 (2015).
Li, L. et al. Hypoxia modulates cell migration and proliferation in placenta-derived mesenchymal stem cells. J. Thorac. Cardiovas. Surg. 154, 543–552.e543 (2017).
Bernardo, MariaE. & Fibbe, WillemE. Mesenchymal stromal cells: sensors and switchers of inflammation. Cell Stem Cell 13, 392–402 (2013).
Elliot, J. et al. Tracey. Bjorkman. Combined mesenchymal stem cell therapy and hypothermia for the treatment of neonatal hypoxic-ischaemic encephalopathy: a systematic review of the preclinical literature. PROSPERO: International prospective register of systematic reviews. 2020. CRD42020196586. Available from: https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=196586
Liberati, A. et al. The Prisma statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 339, b2700 (2009).
Hooijmans, C. R. et al. Syrcle’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 14, 43 (2014).
Zhao, K. et al. Combination of mild therapeutic hypothermia and adipose-derived stem cells for ischemic brain injury. Neural Regen. Res. 13, 1759–1770 (2018).
Bi, M. et al. Bone mesenchymal stem cells transplantation combined with mild hypothermia improves the prognosis of cerebral ischemia in rats. PLoS ONE 13, e0197405 (2018).
Robertson, N. J. et al. Human umbilical cord mesenchymal stromal cells as an adjunct therapy with therapeutic hypothermia in a piglet model of perinatal asphyxia. Cytotherapy 23, 521–535 (2021).
Ahn, S. Y., Chang, Y. S., Sung, D. K., Sung, S. I. & Park, W. S. Hypothermia broadens the therapeutic time window of mesenchymal stem cell transplantation for severe neonatal hypoxic ischemic encephalopathy. Sci. Rep. 8, 7665 (2018).
Park, W. S. et al. Hypothermia augments neuroprotective activity of mesenchymal stem cells for neonatal hypoxic-ischemic encephalopathy. PLoS ONE 10, e0120893 (2015).
Donega, V. et al. Intranasal administration of human MSC for ischemic brain injury in the mouse: in vitro and in vivo neuroregenerative functions. PLoS ONE 9, e112339–e112339 (2014).
Anderson, M. A. et al. Required growth facilitators propel axon regeneration across complete spinal cord injury. Nature 561, 396–400 (2018).
Gu, Y. et al. Conditional ablation of reactive astrocytes to dissect their roles in spinal cord injury and repair. Brain Behav. Immun. 80, 394–405 (2019).
Wagner, W. et al. Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood. Exp. Hematol. 33, 1402–1416 (2005).
Wu, M. et al. Comparison of the biological characteristics of mesenchymal stem cells derived from the human placenta and umbilical cord. Sci. Rep. 8, 5014 (2018).
Talwadekar, M. D., Kale, V. P. & Limaye, L. S. Placenta-derived mesenchymal stem cells possess better immunoregulatory properties compared to their cord-derived counterparts—a paired sample study. Sci. Rep. 5, 15784 (2015).
Weiss, M. L. et al. Immune properties of human umbilical cord Wharton’s Jelly-derived cells. Stem Cells 26, 2865–2874 (2008).
Prasanna, S. J., Gopalakrishnan, D., Shankar, S. R. & Vasandan, A. B. Pro-inflammatory cytokines, ifngamma and tnfalpha, influence immune properties of human bone marrow and Wharton jelly mesenchymal stem cells differentially. PLoS ONE 5, e9016 (2010).
La Rocca, G. et al. Isolation and characterization of Oct-4+/Hla-G+ mesenchymal stem cells from human umbilical cord matrix: differentiation potential and detection of new markers. Histochem. Cell Biol. 131, 267–282 (2009).
Djouad, F. et al. Mesenchymal stem cells inhibit the differentiation of dendritic cells through an interleukin-6-dependent mechanism. Stem Cells 25, 2025–2032 (2007).
Kilkenny, C., Browne, W. J., Cuthill, I. C., Emerson, M. & Altman, D. G. Improving bioscience research reporting: the arrive guidelines for reporting animal research. PLoS Biol. 8, e1000412 (2010).
Ahn, S. Y. et al. Cell type–dependent variation in paracrine potency determines therapeutic efficacy against neonatal hyperoxic lung injury. Cytotherapy 17, 1025–1035 (2015).
Tucker, A. M., Aquilina, K., Chakkarapani, E., Hobbs, C. E. & Thoresen, M. Development of amplitude-integrated electroencephalography and interburst interval in the rat. Pediatr. Res. 65, 62–66 (2009).
Wang, L., Jiang, F., Li, Q., He, X. & Ma, J. Mild hypothermia combined with neural stem cell transplantation for hypoxic-ischemic encephalopathy: neuroprotective effects of combined therapy. Neural. Regen. Res. 9, 1745–1752 (2014). https://doi.org/10.4103/1673-5374.143417.
Funding
E.J.T. and L.E.J. are supported by an Australian Government Research Training Programme Scholarship. S.T.B. and J.A.W. are supported by a National Health and Medical Research Council (NHMRC) Grant. An NHMRC Fellowship supports R.N.B. An NHMRC Practitioner Fellowship supports P.B.C. Funding bodies had no role in study design, data collection and analysis, decision to publish, or manuscript preparation
Author information
Authors and Affiliations
Contributions
E.J.T. conceptualised and designed the study, designed the data collection instruments, collected the data, carried out the analyses, drafted the initial manuscript, and reviewed and revised the manuscript. L.E.J. designed the data collection instruments, collected the data, and revised the manuscript. R.N.B. and P.B.S. reviewed and revised the manuscript. S.T.B. and J.A.W. conceptualised and aided in the study’s design and critically reviewed and revised the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Teo, E.J., Jones, L.E., Wixey, J.A. et al. Combined hypothermia and mesenchymal stem cells in animal models of neonatal hypoxic–ischaemic encephalopathy: a systematic review. Pediatr Res 92, 25–31 (2022). https://doi.org/10.1038/s41390-021-01716-y
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-021-01716-y