Abstract
Background
Metabolic regulation plays a significant role in energy homeostasis, and adolescence is a crucial life stage for the development of cardiometabolic disease (CMD). This study aims to investigate the genetic determinants of metabolic biomarkers—adiponectin, leptin, ghrelin, and orexin—and their associations with CMD risk factors.
Methods
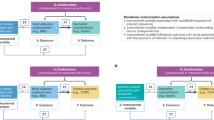
We characterized the genetic determinants of the biomarkers among Hispanic/Latino adolescents of the Santiago Longitudinal Study (SLS) and identified the cumulative effects of genetic variants on adiponectin and leptin using biomarker polygenic risk scores (PRS). We further investigated the direct and indirect effect of the biomarker PRS on downstream body fat percent (BF%) and glycemic traits using structural equation modeling.
Results
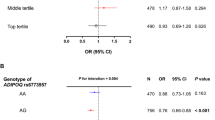
We identified putatively novel genetic variants associated with the metabolic biomarkers. A substantial amount of biomarker variance was explained by SLS-specific PRS, and the prediction was improved by including the putatively novel loci. Fasting blood insulin and insulin resistance were associated with PRS for adiponectin, leptin, and ghrelin, and BF% was associated with PRS for adiponectin and leptin. We found evidence of substantial mediation of these associations by the biomarker levels.
Conclusions
The genetic underpinnings of metabolic biomarkers can affect the early development of CMD, partly mediated by the biomarkers.
Impact
-
This study characterized the genetic underpinnings of four metabolic hormones and investigated their potential influence on adiposity and insulin biology among Hispanic/Latino adolescents.
-
Fasting blood insulin and insulin resistance were associated with polygenic risk score (PRS) for adiponectin, leptin, and ghrelin, with evidence of some degree of mediation by the biomarker levels. Body fat percent (BF%) was also associated with PRS for adiponectin and leptin. This provides important insight on biological mechanisms underlying early metabolic dysfunction and reveals candidates for prevention efforts.
-
Our findings also highlight the importance of ancestrally diverse populations to facilitate valid studies of the genetic architecture of metabolic biomarker levels.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Hales, C. M., Carroll, M. D., Fryar, C. D. & Ogden, C. L. Prevalence of obesity among adults and youth: United States, 2015-2016. NCHS Data Brief 1–8 (2017).
Albala, C., Vio, F., Kain, J. & Uauy, R. Nutrition transition in Chile: determinants and consequences. Public Health Nutr. 5, 123–128 (2002).
Muzzo, S., Burrows, R., Cordero, J. & Ramirez, I. Trends in nutritional status and stature among school-age children in Chile. Nutrition 20, 867–872 (2004).
Ghanemi, A., Yoshioka, M. & St-Amand, J. Broken energy homeostasis and obesity pathogenesis: the surrounding concepts. J. Clin. Med. 7, 453–453 (2018).
Richard, D. Cognitive and autonomic determinants of energy homeostasis in obesity. Nat. Rev. Endocrinol. 11, 489–501 (2015).
Nigro, E. et al. New insight into adiponectin role in obesity and obesity-related diseases. Biomed. Res. Int. 2014, 658913 (2014).
Abella, V. et al. Leptin in the interplay of inflammation, metabolism and immune system disorders. Nat. Rev. Rheumatol. 13, 100–109 (2017).
Chabot, F., Caron, A., Laplante, M. & St-Pierre, D. H. Interrelationships between ghrelin, insulin and glucose homeostasis: physiological relevance. World J. Diabetes 5, 328–341 (2014).
Gray, S. M., Page, L. C. & Tong, J. Ghrelin regulation of glucose metabolism. J. Neuroendocrinol. 31, e12705 (2019).
Klok, M. D., Jakobsdottir, S. & Drent, M. L. The role of leptin and ghrelin in the regulation of food intake and body weight in humans: a review. Obes. Rev. 8, 21–34 (2007).
Poher, A. L., Tschöp, M. H. & Müller, T. D. Ghrelin regulation of glucose metabolism. Peptides 100, 236–242 (2018).
Ebrahim, I. O., Howard, R. S., Kopelman, M. D., Sharief, M. K. & Williams, A. J. The hypocretin/orexin system. J. R. Soc. Med. 95, 227–230 (2002).
Lin, L. et al. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell 98, 365–376 (1999).
Sakurai, T. et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 92, 573–585 (1998).
Heinonen, M. V., Purhonen, A. K., Makela, K. A. & Herzig, K. H. Functions of orexins in peripheral tissues. Acta Physiol 192, 471–485 (2008).
Arihara, Z. et al. Immunoreactive orexin-A in human plasma. Peptides 22, 139–142 (2001).
Cecil, J. E., Tavendale, R., Watt, P., Hetherington, M. M. & Palmer, C. N. An obesity-associated FTO gene variant and increased energy intake in children. N. Engl. J. Med. 359, 2558–2566 (2008).
Llewellyn, C. H., Trzaskowski, M., van Jaarsveld, C. H. M., Plomin, R. & Wardle, J. Satiety mechanisms in genetic risk of obesity. JAMA Pediatr. 168, 338–344 (2014).
Lozoff, B. et al. Behavioral and developmental effects of preventing iron-deficiency anemia in healthy full-term infants. Pediatrics 112, 846–854 (2003).
East, P. et al. Infant iron deficiency, child affect, and maternal unresponsiveness: testing the long-term effects of functional isolation. Dev. Psychol. 53, 2233–2244 (2017).
Pacheco, L. S. et al. Early onset obesity and risk of metabolic syndrome among Chilean adolescents. Prev. Chronic Dis. 14, E93 (2017).
Burrows, R. et al. Healthy Chilean adolescents with HOMA-IR >=2.6 have increased cardiometabolic risk: association with genetic, biological, and environmental factors. J. Diabetes Res. 2015, 783296 (2015).
Lin, D. Y. et al. Genetic association analysis under complex survey sampling: the Hispanic Community Health Study/Study of Latinos. Am. J. Hum. Genet. 95, 675–688 (2014).
Price, A. L. et al. Principal components analysis corrects for stratification in genome-wide association studies. Nat. Genet. 38, 904–909 (2006).
Consortium, G. T. The Genotype-Tissue Expression (GTEx) project. Nat. Genet. 45, 580–585 (2013).
VanderWeele, T. J. Explanation in Causal Inference: Methods for Mediation and Interaction (Oxford University Press, 2015).
VanderWeele, T. J. & Vansteelandt, S. Conceptual issues concerning mediation, interventions and composition. Stat. Interface 2, 457–468 (2009).
Enomoto, T. et al. Adipolin/C1qdc2/CTRP12 protein functions as an adipokine that improves glucose metabolism. J. Biol. Chem. 286, 34552–34558 (2011).
Wei, Z. et al. C1q/TNF-related protein-12 (CTRP12), a novel adipokine that improves insulin sensitivity and glycemic control in mouse models of obesity and diabetes. J. Biol. Chem. 287, 10301–10315 (2012).
Wong, G. W., Wang, J., Hug, C., Tsao, T. S. & Lodish, H. F. A family of Acrp30/adiponectin structural and functional paralogs. Proc. Natl Acad. Sci. USA 101, 10302–10307 (2004).
Chung, C. M. et al. A genome-wide association study reveals a quantitative trait locus of adiponectin on CDH13 that predicts cardiometabolic outcomes. Diabetes 60, 2417–2423 (2011).
Heid, I. M. et al. Clear detection of ADIPOQ locus as the major gene for plasma adiponectin: results of genome-wide association analyses including 4659 European individuals. Atherosclerosis 208, 412–420 (2010).
Morisaki, H. et al. CDH13 gene coding T-cadherin influences variations in plasma adiponectin levels in the Japanese population. Hum. Mutat. 33, 402–410 (2012).
Spracklen, C. N. et al. Exome-derived adiponectin-associated variants implicate obesity and lipid biology. Am. J. Hum. Genet. 105, 15–28 (2019).
Wu, Y. et al. A meta-analysis of genome-wide association studies for adiponectin levels in East Asians identifies a novel locus near WDR11-FGFR2. Hum. Mol. Genet. 23, 1108–1119 (2014).
Dastani, Z. et al. Novel loci for adiponectin levels and their influence on type 2 diabetes and metabolic traits: a multi-ethnic meta-analysis of 45,891 individuals. PLoS Genet. 8, e1002607 (2012).
Qi, L. et al. Novel locus FER is associated with serum HMW adiponectin levels. Diabetes 60, 2197–2201 (2011).
Jee, S. H. et al. Adiponectin concentrations: a genome-wide association study. Am. J. Hum. Genet. 87, 545–552 (2010).
Wu, Y. et al. Genome-wide association study for adiponectin levels in Filipino women identifies CDH13 and a novel uncommon haplotype at KNG1-ADIPOQ. Hum. Mol. Genet. 19, 4955–4964 (2010).
Richards, J. B. et al. A genome-wide association study reveals variants in ARL15 that influence adiponectin levels. PLoS Genet. 5, e1000768 (2009).
Ling, H. et al. Genome-wide linkage and association analyses to identify genes influencing adiponectin levels: the GEMS Study. Obesity 17, 737–744 (2009).
Folkersen, L. et al. Genomic and drug target evaluation of 90 cardiovascular proteins in 30,931 individuals. Nat. Metab. 2, 1135–1148 (2020).
Kilpelainen, T. O. et al. Genome-wide meta-analysis uncovers novel loci influencing circulating leptin levels. Nat. Commun. 7, 10494 (2016).
Yaghootkar, H. et al. Genetic studies of leptin concentrations implicate leptin in the regulation of early adiposity. Diabetes 69, 2806–2818 (2020).
Justice, A. E. et al. Genetic determinants of BMI from early childhood to adolescence: the Santiago Longitudinal Study. Pediatr. Obes. 14, e12479 (2019).
Sargolzaei, J., Chamani, E., Kazemi, T., Fallah, S. & Soori, H. The role of adiponectin and adipolin as anti-inflammatory adipokines in the formation of macrophage foam cells and their association with cardiovascular diseases. Clin. Biochem. 54, 1–10 (2018).
Schaffler, A. & Buechler, C. CTRP family: linking immunity to metabolism. Trends Endocrinol. Metab. 23, 194–204 (2012).
Fadaei, R. et al. Decreased serum levels of CTRP12/adipolin in patients with coronary artery disease in relation to inflammatory cytokines and insulin resistance. Cytokine 113, 326–331 (2019).
Bai, B. et al. Circulating C1q complement/TNF-related protein (CTRP) 1, CTRP9, CTRP12 and CTRP13 concentrations in Type 2 diabetes mellitus: In vivo regulation by glucose. PLoS ONE 12, e0172271 (2017).
Carlson, C. S. et al. Generalization and dilution of association results from European GWAS in populations of non-European ancestry: the PAGE study. PLoS Biol. 11, e1001661 (2013).
Ginsberg, H. N. Insulin resistance and cardiovascular disease. J. Clin. Investig. 106, 453–458 (2000).
Arita, Y. et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. 1999. Biochem. Biophys. Res. Commun. 425, 560–564 (2012).
Tilg, H. & Moschen, A. R. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat. Rev. Immunol. 6, 772–783 (2006).
Borges, M. C. et al. Metabolic profiling of adiponectin levels in adults: Mendelian randomization analysis. Circ. Cardiovasc. Genet. 10, e001837 (2017).
Yaghootkar, H. et al. Mendelian randomization studies do not support a causal role for reduced circulating adiponectin levels in insulin resistance and type 2 diabetes. Diabetes 62, 3589–3598 (2013).
Mente, A. et al. Causal relationship between adiponectin and metabolic traits: a Mendelian randomization study in a multiethnic population. PLoS ONE 8, e66808 (2013).
Gao, H. et al. Evidence of a causal relationship between adiponectin levels and insulin sensitivity: a Mendelian randomization study. Diabetes 62, 1338–1344 (2013).
Nielsen, M. B., Colak, Y., Benn, M. & Nordestgaard, B. G. Causal relationship between plasma adiponectin and body mass index: one- and two-sample bidirectional Mendelian randomization analyses in 460,397 individuals. Clin. Chem. https://doi.org/10.1093/clinchem/hvaa227 (2020).
Farooqi, I. S. et al. Effects of recombinant leptin therapy in a child with congenital leptin deficiency. N. Engl. J. Med. 341, 879–884 (1999).
Myers, M. G., Cowley, M. A. & Munzberg, H. Mechanisms of leptin action and leptin resistance. Annu. Rev. Physiol. 70, 537–556 (2008).
Zhang, C. S. et al. The correlation between circulating ghrelin and insulin resistance in obesity: a meta-analysis. Front. Physiol. 9, 1308 (2018).
van Jaarsveld, C. H., Boniface, D., Llewellyn, C. H. & Wardle, J. Appetite and growth: a longitudinal sibling analysis. JAMA Pediatr. 168, 345–350 (2014).
van Jaarsveld, C. H., Llewellyn, C. H., Johnson, L. & Wardle, J. Prospective associations between appetitive traits and weight gain in infancy. Am. J. Clin. Nutr. 94, 1562–1567 (2011).
Parkinson, K. N., Drewett, R. F., Le Couteur, A. S., Adamson, A. J. & Gateshead Milennium Study Core Team. Do maternal ratings of appetite in infants predict later Child Eating Behaviour Questionnaire scores and body mass index? Appetite 54, 186–190 (2010).
Acknowledgements
We thank the participants of the Santiago Longitudinal Study, the San Antonio Family Heart Study and the San Antonio Family Diabetes/Gallbladder Study for their continued cooperation and participation in our research programs.
Funding
This work was funded in part by University of North Carolina Nutrition Research Institute internal pilot grant, AHA grant 15GRNT25880008, and NIH award K99/R00HL130580-02. We thank the participants and their family members from the Santiago Longitudinal Study (SLS) (R01 HL088530, R01 HD33487). K.E.N. is additionally supported by R01HL151152 and R01 DK122503. Work for the validation study was supported in part by National Institutes of Health (NIH) grants P01 HL045522, R01 DK047482, DK053889, R01 HL113323, R37 MH059490, and T2D-GENES Consortium grants (U01 DK085524, U01 DK085584, U01 DK085501, U01 DK085526, and U01 DK085545).
Author information
Authors and Affiliations
Contributions
D.K. and K.E.N. designed the study and drafted the initial manuscript; E.B. and R.B. collected the data; A.E.J., G.C., M.G., and Y.W. carried out genetic data cleaning; D.K., M.G., A.G.H., Y.W., R.R., and V.L.B. conducted statistical analysis; M.A., J.P., D.M.L., J.E.C., A.G.C., R.D., and J.B. were involved in the validation study; D.K., K.E.N., M.G., A.G.H., A.E.J., and G.C. were involved in interpretation of the results; all authors revised the manuscript and contributed to the content and approved the submission and publication of the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The research conducted herein constitutes an analysis of de-identified existing data and therefore is not considered human subject research.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Kim, D., Justice, A.E., Chittoor, G. et al. Genetic determinants of metabolic biomarkers and their associations with cardiometabolic traits in Hispanic/Latino adolescents. Pediatr Res 92, 563–571 (2022). https://doi.org/10.1038/s41390-021-01729-7
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-021-01729-7