Abstract
Background
To investigate mechanisms of injury and recovery in neonatal encephalopathy (NE), we performed targeted metabolomic analysis of plasma using liquid chromatography with tandem mass spectrometry (LC/MS/MS) from healthy term neonates or neonates with NE.
Methods
Plasma samples from the NE (n = 45, day of life 0–1) or healthy neonatal (n = 30, ≥36 weeks gestation) cohorts had LC/MS/MS metabolomic profiling with a 193-plex targeted metabolite assay covering >366 metabolic pathways. Metabolite levels were compared to 2-year neurodevelopmental outcomes measured by the Bayley Scales of Infant and Toddler Development III (Bayley-III).
Results
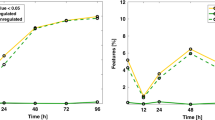
Out of 193 metabolites, 57 met the pre-defined quality control criteria for analysis. Significant (after false discovery rate correction) KEGG (Kyoto Encyclopedia of Genes and Genomes) pathways included aminoacyl-tRNA biosynthesis, arginine biosynthesis, and metabolism of multiple amino acids. Significant disease pathways included seizures. In regression models, histidine and C6 sugar amine were significantly associated with cognitive, motor, and language and betaine with cognitive and motor Bayley-III composite scores. The addition of histidine, C6 sugar amine, and betaine to a Sarnat score-based clinical regression model significantly improved model performance (Akaike information criterion and adjusted r2) for Bayley-III cognitive, motor, and language scores.
Conclusions
Plasma metabolites may help to predict neurological outcomes in neonatal brain injury and enhance current clinical predictors.
Impact
-
Plasma metabolites may help to predict neurological outcomes in NE and supplement current clinical predictors.
-
Current metabolomics research is limited in terms of clinical application and association with long-term outcomes.
-
Our study presents novel associations of plasma metabolites from the first 24 h of life and 2-year neurodevelopmental outcomes for infants with NE.
-
Our metabolomics discovery provides insight into possible disease mechanisms and methods to rescue and/or supplement metabolic pathways involved in NE.
-
Our metabolomics discovery of metabolic pathway supplementations and/or rescue mechanisms may serve as adjunctive therapies for NE.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Kurinczuk, J. J., White-Koning, M. & Badawi, N. Epidemiology of neonatal encephalopathy and hypoxic–ischaemic encephalopathy. Early Hum. Dev. 86, 329–338 (2010).
Molloy, E. J. & Bearer, C. Neonatal encephalopathy versus hypoxic-ischemic encephalopathy. Pediatr. Res. 84, 574 (2018).
Volpe, J. J. Neonatal encephalopathy: an inadequate term for hypoxic-ischemic encephalopathy. Ann. Neurol. 72, 156–166 (2012).
Shankaran, S. et al. Effect of depth and duration of cooling on death or disability at age 18 months among neonates with hypoxic-ischemic encephalopathy a randomized clinical trial. JAMA 318, 57–67 (2017).
Wyatt, J. S. et al. Determinants of outcomes after head cooling for neonatal encephalopathy. Pediatrics 119, 912–921 (2007).
Gluckman, P. D. et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet 365, 663–670 (2005).
Ferriero, D. M. Neonatal brain injury. N. Engl. J. Med. 352, 839–839 (2005).
Gerner, G. J. et al. Transfontanellar duplex brain ultrasonography resistive indices as a prognostic tool in neonatal hypoxic-ischemic encephalopathy before and after treatment with therapeutic hypothermia. J. Perinatol. 36, 202–206 (2016).
American College of Obstetricians and Gynecologists’ Task Force on Neonatal Encephalopathy. Neonatal encephalopathy and neurologic outcome, second edition. Obstet. Gynecol. 123, 896–901 (2014).
Barkovich, A. J. et al. MR imaging, MR spectroscopy, and diffusion tensor imaging of sequential studies in neonates with encephalopathy. Am. J. Neuroradiol. 27, 533–547 (2006).
McKinstry, R. C. et al. A prospective, longitudinal diffusion tensor imaging study of brain injury in newborns. Neurology 59, 824–833 (2002).
Graham, E. M., Everett, A. D., Delpech, J. C. & Northington, F. J. Blood biomarkers for evaluation of perinatal encephalopathy: state of the art. Curr. Opin. Pediatr. 30, 199–203 (2018).
Nelson, K. et al. Antecedents of neonatal encephalopathy in the Vermont Oxford Network Encephalopathy Registry. Pediatrics 130, 878–886 (2012).
Douglas-Escobar, M. & Weiss, M. D. Hypoxic-ischemic encephalopathy: a review for the clinician. JAMA Pediatr. 169, 397–403 (2015).
Wagner, S. R. & Lanier, W. L. Metabolism of glucose, glycogen, and high-energy phosphates during complete cerebral ischemia: a comparison of normoglycemic, chronically hyperglycemic diabetic, and acutely hyperglycemic nondiabetic rats. Anesthesiology 81, 1516–1526 (1994).
Smith, M. L., Von Hanwehr, R. & Siesjo, B. K. Changes in extra- and intracellular pH in the brain during and following ischemia in hyperglycemic and in moderately hypoglycemic rats. J. Cereb. Blood Flow. Metab. 6, 574–583 (1986).
Efstathiou, N., Theodoridis, G. & Sarafidis, K. Understanding neonatal hypoxic-ischemic encephalopathy with metabolomics. Hippokratia 21, 115–123 (2017).
Sánchez-illana, Á. et al. Evolution of energy related metabolites in plasma from newborns with hypoxic-ischemic encephalopathy during hypothermia treatment. Sci. Rep. 7, 1–12 (2017).
Piñeiro-Ramos, J. D. et al. Metabolic phenotypes of hypoxic-ischemic encephalopathy with normal vs pathologic magnetic resonance imaging outcomes. Metabolites 10, 109 (2020).
Piñeiro-Ramos, J. D. et al. Noninvasive monitoring of evolving urinary metabolic patterns in neonatal encephalopathy. Pediatr. Res. https://doi.org/10.1038/s41390-021-01553-z, 1–8 (2021).
Sarnat, H. B. & Sarnat, M. S. Encephalopathy fetal distress: a clinical and and electroencephalographic study. Arch. Neurol. 33, 696–705 (1976).
Sweetman, D. et al. Coagulation profiles are associated with early clinical outcomes in neonatal encephalopathy. Front. Pediatr. 7, 1–7 (2019).
O’Hare, F. M. et al. Serial cytokine alterations and abnormal neuroimaging in newborn infants with encephalopathy. Acta Paediatr. Int. J. Paediatr. 106, 561–567 (2017).
Bayley, N. Bayley Scales of Infant and Toddler Development Manual 3rd edn (The Pscyhological Corporation, 2006).
Shankaran, S. et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N. Engl. J. Med. 353, 1574–1584 (2005).
Tobin, J. Estimation of relationships for limited dependent variables. Econometrica 26, 24–36 (1958).
R Core Team. R: A Language and Environment for Statistical Computing (R Core Team, 2019).
Pang, Z., Chong, J., Li, S. & Xia, J. Metaboanalystr 3.0: Toward an optimized workflow for global metabolomics. Metabolites 10, 186 (2020).
Rainesalo, S. et al. Plasma and cerebrospinal fluid amino acids in epileptic patients. Neurochem. Res. 29, 319–324 (2004).
Schnaar, R. L., Gerardy-Schahn, R. & Hildebrandt, H. Sialic acids in the brain: gangliosides and polysialic acid in nervous system development, stability, disease, and regeneration. Physiol. Rev. 94, 461–518 (2014).
van Karnebeek, C. D. M. et al. NANS-mediated synthesis of sialic acid is required for brain and skeletal development. Nat. Genet. 48, 777–784 (2016).
Wen, X. Y. et al. Sialic acid catabolism by N-acetylneuraminate pyruvate lyase is essential for muscle function. JCI insight 3, e122373 (2018).
Lisanti, M. P., Field, M. C., Caras, I. W., Menon, A. K. & Rodriguez-Boulan, E. Mannosamine, a novel inhibitor of glycosyl-phosphatidylinositole incorporation into proteins. EMBO J. 10, 1969–1977 (1991).
Holecek, M. Histidine in health and disease: metabolism, physiological importance, and use as a supplement. Nutrients 12, 1–20 (2020).
Ishikawa, M. Developmental disorders in histidinemia - follow-up study of language development in histidinemia. Acta Paediatr. Jpn. 29, 224–228 (1987).
Scriver, C. R. & Levy, H. L. Histidinaemia. Part I: reconciling retrospective and prospective findings. J. Inherit. Metab. Dis. 6, 51–53 (1983).
Locasale, J. W. Serine, glycine and one-carbon units: cancer metabolism in full circle. Nat. Rev. Cancer 13, 572–583 (2013).
Zhao, G. et al. Betaine in inflammation: mechanistic aspects and applications. Front. Immunol. 9, 1–13 (2018).
Chitturi, J., Li, Y., Santhakumar, V. & Kannurpatti, S. S. Early behavioral and metabolomic change after mild to moderate traumatic brain injury in the developing brain. Neurochem. Int. 120, 75–86 (2018).
Locci, E. et al. Exploring perinatal asphyxia by metabolomics. Metabolites 10, 1–19 (2020).
Sánchez-illana, Á., Piñeiro-ramos, J. D. & Kuligowski, J. Seminars in fetal and neonatal medicine small molecule biomarkers for neonatal hypoxic ischemic encephalopathy. Semin. Fetal Neonatal Med. 25, 101084 (2020).
Laboratories, K. Glycine, serine and threonine metabolism - reference pathway. KEGG Pathway https://www.genome.jp/pathway/map00260 (2019).
Buchman, A. L. The addition of choline to parenteral nutrition. Gastroenterology 137, S119–S128 (2009).
Locci, E. et al. A longitudinal 1 H-NMR metabolomics analysis of urine from newborns with hypoxic- ischemic encephalopathy undergoing hypothermia therapy. Clinical and medical legal insights. PLoS ONE 13, e0194267 (2018).
Andrade, E., Chavez, W., Shaikh, Z. I. & Torres, A. R. Neonatal encephalopathies: a clinical perspective. Cureus 11, e4948 (2019).
Kharoshankaya, L. et al. Seizure burden and neurodevelopmental outcome in neonates with hypoxic–ischemic encephalopathy. Dev. Med. Child Neurol. 58, 1242–1248 (2016).
Wusthoff, C. J. et al. Electrographic seizures during therapeutic hypothermia for neonatal hypoxic-ischemic encephalopathy. J. Child Neurol. 26, 724–728 (2011).
Badawy, A. A. B. Kynurenine pathway of tryptophan metabolism: regulatory and functional aspects. Int. J. Tryptophan Res. 10, 1178646917691938 (2017).
Lovelace, M. D. et al. Recent evidence for an expanded role of the kynurenine pathway of tryptophan metabolism in neurological diseases. Neuropharmacology 112, 373–388 (2017).
Chen, W. C. et al. Aryl hydrocarbon receptor modulates stroke-induced astrogliosis and neurogenesis in the adult mouse brain. J. Neuroinflamm. 16, 1–13 (2019).
Sweetman, D. U. et al. Neonatal encephalopathy is associated with altered IL-8 and GM-CSF which correlates with outcomes. Front. Pediatr. 8, 1–7 (2021).
Zareen, Z. et al. Cytokine dysregulation persists in childhood post neonatal encephalopathy. BMC Neurol. 20, 1–9 (2020).
Acknowledgements
We thank the patients and their families for their participation and contributions to this study. We also thank the Everett research group at Johns Hopkins University School of Medicine for their support and contributions. We thank the Johns Hopkins University School of Medicine Scholarly Concentration mentor Dr. Meredith Atkinson and the Johns Hopkins University School of Medicine Dean’s Funding for their support and contributions.
Funding
This work was supported by NIH NICHD R01HD086058 (to A.D.E. and F.N.); Health Research Board, Ireland; Trinity College Dublin; National Children’s Research Centre.
Author information
Authors and Affiliations
Contributions
Substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data: B.D.F., E.M., T.S., J.Z., M.S., V.D., D.S., L.K., M.O’D., A.R., R.H., G.E., C.M., D.G., F.N. and A.D.E. Drafting the article or revising it critically for important intellectual content: B.D.F., E.M., A.R., R.H., C.M., D.G., F.N. and A.D.E. Final approval of the version to be published: B.D.F., E.M., T.S., J.Z., M.S., V.D., D.S., L.K., M.O’D., A.R., R.H., G.E., C.M., D.G., F.N. and A.D.E.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent statement
Signed informed consent was obtained from the parent of each participant.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Friedes, B.D., Molloy, E., Strickland, T. et al. Neonatal encephalopathy plasma metabolites are associated with neurodevelopmental outcomes. Pediatr Res 92, 466–473 (2022). https://doi.org/10.1038/s41390-021-01741-x
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-021-01741-x
This article is cited by
-
Meconium metabolomic profiling dysregulation and neonatal brain injury in selective fetal growth restriction
BMC Pregnancy and Childbirth (2026)
-
Biomarkers in neonatal encephalopathy: the role of high-mobility group box 1 in prognosis and potential therapy
Pediatric Research (2025)
-
Searching molecular biomarkers correlating with BSID-III at 24 months in infants with neonatal hypoxic-ischemic encephalopathy
European Journal of Pediatrics (2024)