Abstract
Background
l-Asparaginase (l-Asp) is an important therapeutic for childhood acute lymphoblastic leukemia (ALL). Asparaginase-associated pancreatitis (AAP) is a severe complication of l-Asp related to the dosage. We investigated the incidence of, and risk factors for, AAP in pediatric patients with ALL.
Methods
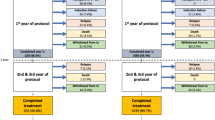
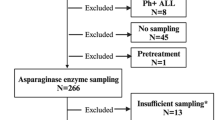
From January 2002 to December 2018, pediatric patients with ALL treated at National Taiwan University Hospital were enrolled in this study. The diagnosis of AAP was based on the criteria of the Ponte di Legno Toxicity Working Group.
Results
Of the 353 patients enrolled in this study, 14 (4.0%) developed AAP. The incidence of AAP in ALL patients was significantly higher after treatment with the 2013 protocol compared with the 2002 protocol of the Taiwan Pediatric Oncology Group (9.5% vs. 1.3%). Multivariate analysis showed that a high peak l-Asp dose intensity (>45,000 U/m2/month) and older age at diagnosis (>6.8 years) were independently predictive of AAP development.
Conclusions
The incidence of acute pancreatitis in childhood ALL was correlated more strongly with the peak dose intensity than with the cumulative dose of l-Asp. These results could be used to reduce the treatment-related complications of ALL.
Impact
-
l-Asparaginase is an important therapeutic for childhood acute lymphoblastic leukemia, and the accumulated dosage of l-asparaginase is considered as a major risk factor of asparaginase-associated pancreatitis.
-
This article demonstrated that the incidence of pancreatitis correlates with the dose-intensity of l-asparaginase, but not the accumulated dosage.
-
Identification of patient group with high risk of pancreatitis could lead to early diagnosis and reduce the complication.
-
This finding could aid in developing further new protocol or therapeutic strategy design to reduce treatment-related complications and improve clinical outcomes of childhood acute lymphoblastic leukemia.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Suzuki, M., Sai, J. K. & Shimizu, T. Acute pancreatitis in children and adolescents. World J. Gastrointest. Pathophysiol. 5, 416–426 (2014).
Tallal, L. et al. E. coli L-asparaginase in the treatment of leukemia and solid tumors in 131 children. Cancer 25, 306–320 (1970).
Pui, C. H., Mullighan, C. G., Evans, W. E. & Relling, M. V. Pediatric acute lymphoblastic leukemia: where are we going and how do we get there? Blood 120, 1165–1174 (2012).
Prucker, C. et al. Induction death and treatment-related mortality in first remission of children with acute lymphoblastic leukemia: a population-based analysis of the Austrian Berlin-Frankfurt-Munster Study Group. Leukemia 23, 1264–1269 (2009).
Silverman, L. B. et al. Improved outcome for children with acute lymphoblastic leukemia: results of Dana-Farber Consortium Protocol 91-01. Blood 97, 1211–1218 (2001).
Gupta, S. et al. Impact of asparaginase discontinuation on outcome in childhood acute lymphoblastic leukemia: a report from the Children’s Oncology Group. J. Clin. Oncol. 38, 1897–1905 (2020).
Liu, C. et al. Clinical and genetic risk factors for acute pancreatitis in patients with acute lymphoblastic leukemia. J. Clin. Oncol. 34, 2133–2140 (2016).
Raja, R. A. et al. Asparaginase-associated pancreatitis in children with acute lymphoblastic leukaemia in the Nopho All2008 protocol. Br. J. Haematol. 165, 126–133 (2014).
Moghrabi, A. et al. Results of the Dana-Farber Cancer Institute all consortium protocol 95-01 for children with acute lymphoblastic leukemia. Blood 109, 896–904 (2007).
Sakaguchi, S., Higa, T., Suzuki, M., Fujimura, J. & Shimizu, T. Prophylactic use of octreotide for asparaginase-induced acute pancreatitis. Int. J. Hematol. 106, 266–268 (2017).
Wolthers, B. O. et al. Asparaginase-associated pancreatitis: a study on phenotype and genotype in the Nopho All2008 Protocol. Leukemia 31, 325–332 (2017).
Kearney, S. L. et al. Clinical course and outcome in children with acute lymphoblastic leukemia and asparaginase-associated pancreatitis. Pediatr. Blood Cancer 53, 162–167 (2009).
Samarasinghe, S. et al. Incidence and outcome of pancreatitis in children and young adults with acute lymphoblastic leukaemia treated on a contemporary protocol, Ukall 2003. Br. J. Haematol. 162, 710–713 (2013).
Liang, D. C. et al. Long-term results of Taiwan Pediatric Oncology Group Studies 1997 and 2002 for childhood acute lymphoblastic leukemia. Leukemia 24, 397–405 (2010).
Yeh, T. C. et al. Treatment of childhood acute lymphoblastic leukemia with delayed first intrathecal therapy and omission of prophylactic cranial irradiation: results of the Tpog-All-2002 Study. Cancer 124, 4538–4547 (2018).
Wolthers, B. O. et al. Asparaginase-associated pancreatitis in childhood acute lymphoblastic leukaemia: an observational Ponte Di Legno Toxicity Working Group Study. Lancet Oncol. 18, 1238–1248 (2017).
Ben Tanfous, M. et al. Polymorphisms of asparaginase pathway and asparaginase-related complications in children with acute lymphoblastic leukemia. Clin. Cancer Res. 21, 329–334 (2015).
Amylon, M. D. et al. Intensive high-dose asparaginase consolidation improves survival for pediatric patients with T cell acute lymphoblastic leukemia and advanced stage lymphoblastic lymphoma: A Pediatric Oncology Group Study. Leukemia 13, 335–342 (1999).
Pession, A. et al. Long-term results of a randomized trial on extended use of high dose L-asparaginase for standard risk childhood acute lymphoblastic leukemia. J. Clin. Oncol. 23, 7161–7167 (2005).
Hijiya, N. & van der Sluis, I. M. Asparaginase-associated toxicity in children with acute lymphoblastic leukemia. Leuk. Lymphoma 57, 748–757 (2016).
Pieters, R. et al. L-Asparaginase treatment in acute lymphoblastic leukemia: a focus on Erwinia asparaginase. Cancer 117, 238–249 (2011).
Asselin, B. L. et al. Measurement of serum L-asparagine in the presence of L-asparaginase requires the presence of an L-asparaginase inhibitor. Cancer Res. 51, 6568–6573 (1991).
Asselin, B. & Rizzari, C. Asparaginase pharmacokinetics and implications of therapeutic drug monitoring. Leuk. Lymphoma 56, 2273–2280 (2015).
Duval, M. et al. Comparison of Escherichia coli-asparaginase with Erwinia-asparaginase in the treatment of childhood lymphoid malignancies: results of a randomized European Organisation for Research and Treatment of Cancer-Children’s Leukemia Group Phase 3 Trial. Blood 99, 2734–2739 (2002).
Raja, R. A., Schmiegelow, K. & Frandsen, T. L. Asparaginase-associated pancreatitis in children. Br. J. Haematol. 159, 18–27 (2012).
Stock, W. et al. Prevention and management of asparaginase/pegasparaginase-associated toxicities in adults and older adolescents: recommendations of an Expert Panel. Leuk. Lymphoma 52, 2237–2253 (2011).
Alvarez, O. A. & Zimmerman, G. Pegaspargase-induced pancreatitis. Med. Pediatr. Oncol. 34, 200–205 (2000).
Panetta, J. C. et al. Comparison of native E. coli and Peg asparaginase pharmacokinetics and pharmacodynamics in pediatric acute lymphoblastic leukemia. Clin. Pharmacol. Ther. 86, 651–658 (2009).
Place, A. E. et al. Intravenous pegylated asparaginase versus intramuscular native Escherichia coli L-asparaginase in newly diagnosed childhood acute lymphoblastic leukaemia (Dfci 05-001): a randomised, open-label phase 3 trial. Lancet Oncol. 16, 1677–1690 (2015).
Abaji, R. et al. Whole-exome sequencing identified genetic risk factors for asparaginase-related complications in childhood all patients. Oncotarget 8, 43752–43767 (2017).
Wolthers, B. O. et al. Trypsin-encoding Prss1-Prss2 variations influence the risk of asparaginase-associated pancreatitis in children with acute lymphoblastic leukemia: a Ponte Di Legno Toxicity Working Group Report. Haematologica 104, 556–563 (2019).
Mukherjee, A. et al. Asparagine synthetase is highly expressed at baseline in the pancreas through heightened perk signaling. Cell Mol. Gastroenterol. Hepatol. 9, 1–13 (2020).
Rank, C. U. et al. Asparaginase-associated pancreatitis in acute lymphoblastic leukemia: results from the Nopho All2008 treatment of patients 1-45 years of age. J. Clin. Oncol. 38, 145–154 (2020).
Cooper, S. L. et al. Universal premedication and therapeutic drug monitoring for asparaginase-based therapy prevents infusion-associated acute adverse events and drug substitutions. Pediatr. Blood Cancer 66, e27797 (2019).
Funding
This study is supported by grants from the Ministry of Science and Technology, Taiwan (MOST) (109-2314-B-002-129, 110-2314-B-002-088-MY3 [to H-H-C.]). The remaining authors received no external funding. The funder did not participate in the work.
Author information
Authors and Affiliations
Contributions
C.-B.C., the first author of the study, is responsible for the study design, data management, manuscript writing, and data analysis. H.-H.C. and J.-F.W. are responsible for long-term patients follow, recruitment, study design, critical review of the article, and is the principal investigator of this study. S-W.C., Y.-L.Y., M.-Y.L., S.-T.J., H.-L.C., Y.-H.N, D.-T.L., and M.-H.C. are responsible for long-term patients follow, recruitment, and critical review of the manuscript. All authors have seen and approved the final version. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Chen, CB., Chang, HH., Chou, SW. et al. Acute pancreatitis in children with acute lymphoblastic leukemia correlates with L-asparaginase dose intensity. Pediatr Res 92, 459–465 (2022). https://doi.org/10.1038/s41390-021-01796-w
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41390-021-01796-w
This article is cited by
-
Pancreatitis associated with BRAF inhibitors: a disproportionality analysis based on the Food and Drug Administration Adverse Event Reporting System
International Journal of Clinical Pharmacy (2025)
-
Prediction of chemotherapy-related complications in pediatric oncology patients: artificial intelligence and machine learning implementations
Pediatric Research (2023)
-
Does L-asparaginase dose intensity correlate with acute pancreatitis in acute lymphoblastic leukemia patients?
Pediatric Research (2022)