Abstract
Background
To determine the associations of urinary CXC motif chemokine 10 (uCXCL10) with AKI, sepsis and pediatric intensive care unit (PICU) mortality in critically ill children, as well as its predictive value for the aforementioned issues.
Methods
Urinary CXCL10 levels were serially measured in 342 critically ill children during the first week after PICU admission. AKI diagnosis was based on the criteria of KDIGO. Sepsis was diagnosed according to the surviving sepsis campaign’s international guidelines for children.
Results
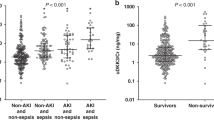
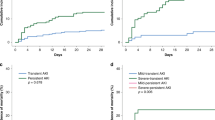
Fifty-two (15.2%) children developed AKI, 132 (38.6%) were diagnosed with sepsis, and 30 (12.3%) died during the PICU stay. Both the initial and peak values of uCXCL10 remained independently associated with AKI, sepsis, septic AKI and PICU mortality. The AUCs of the initial uCXCL10 for predicting AKI, sepsis, septic AKI and PICU mortality were 0.63 (0.53–0.72), 0.62 (0.56–0.68), 0.75 (0.64–0.87) and 0.77 (0.68–0.86), respectively. The AUCs for prediction by using peak uCXCL10 were as follows: AKI 0.65 (0.56–0.75), sepsis 0.63 (0.57–0.69), septic AKI 0.76 (0.65–0.87) and PICU mortality 0.84 (0.76–0.91).
Conclusions
Urinary CXCL10 is independently associated with AKI and sepsis and may be a potential indicator of septic AKI and PICU mortality in critically ill children.
Impact
-
Urinary CXC motif chemokine 10 (uCXCL10), as an inflammatory mediator, has been proposed to be a biomarker for AKI in a specific setting. AKI biomarkers are often susceptible to confounding factors, limiting their utility as a specific biomarker, especially in heterogeneous population.
-
This study revealed that uCXCL10 levels are independently associated with increased risk for AKI, sepsis, septic AKI and PICU mortality.
-
A higher uCXCL10 may be predictive of septic AKI and PICU mortality in critically ill children.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Ronco, C., Bellomo, R. & Kellum, J. A. Acute kidney injury. Lancet. 394, 1949–1964 (2019).
Hoste, E. A. J. et al. Global epidemiology and outcomes of acute kidney injury. Nat. Rev. Nephrol. 14, 607–625 (2018).
Noble, R. A., Lucas, B. J. & Selby, N. M. Long-term outcomes in patients with acute kidney injury. Clin. J. Am. Soc. Nephrol. 15, 423–429 (2020).
Kaddourah, A. et al. Epidemiology of acute kidney injury in critically ill children and young adults. N. Engl. J. Med. 376, 11–20 (2017).
Kari, J. A. et al. Outcome of pediatric acute kidney injury: a multicenter prospective cohort study. Pediatr. Nephrol. 33, 335–340 (2018).
Greenberg, J. H. & Parikh, C. R. Biomarkers for diagnosis and prognosis of AKI in children: one size does not fit all. Clin. J. Am. Soc. Nephrol. 12, 1551–1557 (2017).
Hanna, M. et al. Early urinary biomarkers of acute kidney injury in preterm infants. Pediatr. Res. 80, 218–223 (2016).
Ortiz, A. et al. Mitogen-activated protein kinase 14 promotes AKI. J. Am. Soc. Nephrol. 28, 823–836 (2017).
Fiorina, P. et al. Role of CXC chemokine receptor 3 pathway in renal ischemic injury. J. Am. Soc. Nephrol. 17, 716–723 (2006).
Neville, L. F., Mathiak, G. & Bagasra, O. The immunobiology of interferon-gamma inducible protein 10 kD (IP-10): a novel, pleiotropic member of the C-X-C chemokine superfamily. Cytokine Growth Factor Rev. 8, 207–219 (1997).
Narumi, S. et al. Tissue-specific expression of murine IP-10 mRNA following systemic treatment with interferon gamma. J. Leukoc. Biol. 52, 27–33 (1992).
Cockwell, P. et al. Chemoattraction of T cells expressing CCR5, CXCR3 and CX3CR1 by proximal tubular epithelial cell chemokines. Nephrol. Dial. Transpl. 17, 734–744 (2002).
Hu, H. et al. Elevation of CXCR3-binding chemokines in urine indicates acute renal-allograft dysfunction. Am. J. Transpl. 4, 432–437 (2004).
Ho, J. et al. Mass spectrometry-based proteomic analysis of urine in acute kidney injury following cardiopulmonary bypass: a nested case-control study. Am. J. Kidney Dis. 53, 584–595 (2009).
Erez, D. L. et al. Acute kidney injury in children after hematopoietic cell transplantation is associated with elevated urine CXCL10 and CXCL9. Biol. Blood Marrow Transpl. 26, 1266–1272 (2020).
Vaidya, V. S. et al. Urinary biomarkers for sensitive and specific detection of acute kidney injury in humans. Clin. Transl. Sci. 1, 200–208 (2008).
Hoste, E. A. et al. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med. 41, 1411–1423 (2015).
Chan, T. & Gu, F. Early diagnosis of sepsis using serum biomarkers. Expert Rev. Mol. Diagn. 11, 487–496 (2011).
Jekarl, D. W. et al. Diagnosis and prognosis of sepsis based on use of cytokines, chemokines, and growth factors. Dis. Markers. 2019, 1089107 (2019).
Goldstein, B., Giroir, B. & Randolph, A., International Consensus Conference on Pediatric S. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr. Crit. Care Med. 6, 2–8 (2005).
Fang, F. et al. Subclinical acute kidney injury is associated with adverse outcomes in critically ill neonates and children. Crit. Care 22, 256 (2018).
Khwaja, A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin. Pract. 120, c179–184 (2012).
Chen, J. et al. Association of fluid accumulation with clinical outcomes in critically ill children with severe sepsis. PLoS ONE. 11, e0160093 (2016).
Weiss, S. L. et al. Executive summary: surviving sepsis campaign international guidelines for the management of septic shock and sepsis-associated organ dysfunction in children. Intensive Care Med. 46, 1–9 (2020).
Matics, T. J. & Sanchez-Pinto, L. N. Adaptation and validation of a Pediatric Sequential Organ Failure Assessment Score and evaluation of the Sepsis-3 definitions in critically ill children. JAMA Pediatr. 171, e172352 (2017).
Gao, J., Wu, L., Wang, S. & Chen, X. Role of chemokine (C-X-C Motif) ligand 10 (CXCL10) in renal diseases. Mediators Inflamm. 2020, 6194864 (2020).
Kirita, Y. et al. Cell profiling of mouse acute kidney injury reveals conserved cellular responses to injury. Proc. Natl Acad. Sci USA. 117, 15874–15883 (2020).
Poston, J. T. & Koyner, J. L. Sepsis associated acute kidney injury. BMJ. 364, k4891 (2019).
Herzig, D. S. et al. The role of CXCL10 in the pathogenesis of experimental septic shock. Crit Care. 18, R113 (2014).
Ng, P. C. et al. IP-10 is an early diagnostic marker for identification of late-onset bacterial infection in preterm infants. Pediatr Res. 61, 93–98 (2007).
Maier, S. et al. Massive chemokine transcription in acute renal failure due to polymicrobial sepsis. Shock. 14, 187–192 (2000).
Bellomo, R. et al. Acute kidney injury in sepsis. Intensive Care Med. 43, 816–828 (2017).
Fiorentino, M. et al. Serial measurement of cell-cycle arrest biomarkers [TIMP-2]*[IGFBP7] and risk for progression to death, dialysis or severe acute kidney injury in patients with septic shock. Am J Respir Crit Care Med. 202, 1262–1270 (2020).
Zhang, A. et al. Diagnosis and prognosis of neutrophil gelatinase-associated lipocalin for acute kidney injury with sepsis: a systematic review and meta-analysis. Crit Care 20, 41 (2016).
Lehmann, J. M. et al. Circulating CXCL10 in cirrhotic portal hypertension might reflect systemic inflammation and predict ACLF and mortality. Liver Int. 38, 875–884 (2018).
Landreneau, M. J. et al. CCL2 and CXCL10 are associated with poor outcome after intracerebral hemorrhage. Ann Clin Transl Neurol. 5, 962–970 (2018).
Kwon, O. et al. Simultaneous monitoring of multiple urinary cytokines may predict renal and patient outcome in ischemic AKI. Ren Fail. 32, 699–708 (2010).
Rudd, K. E. et al. Global, regional, and national sepsis incidence and mortality, 1990-2017: analysis for the Global Burden of Disease Study. Lancet. 395, 200–211 (2020).
Pickering, J. W. & Endre, Z. H. Back-calculating baseline creatinine with MDRD misclassifies acute kidney injury in the intensive care unit. Clin J Am Soc Nephrol. 5, 1165–1173 (2010).
Watson, D. et al. A novel multi-biomarker assay for non-invasive quantitative monitoring of kidney injury. J Clin Med. 8, 499 (2019).
Acknowledgements
We thank the staff in biochemistry laboratory for technical assistance.
Funding
This work was supported by grants from the National Natural Science Foundation of China (81971432), Jiangsu Province Science and Technology Support Program (BE2020660), Natural Science Foundation of Jiangsu Province (BK20171217), Key Talent of Women’s and Children’s Health of Jiangsu Province (FRC201738). The funders had no role in study design, data collection, preparation of the manuscript, and decision to publish.
Author information
Authors and Affiliations
Contributions
H.H. performed the experiments and data analysis and drafted the manuscript. H.Z. participated in data analysis and revised the manuscript. W.W., J.C., and Z.B. had primary responsibility for diagnosis of sepsis and participated in clinical data collection. X.D. and W.L. participated in collecting the data and samples. J.P. and X.L. participated in data analysis and interpretation. J.W. participated in the design of the study and coordination. Y.L. had primary responsibility for study design, performing the experiments, data analysis, interpretation of data, and writing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The study was approved by the Institutional Review Board of the Children’s Hospital of Soochow university, and parental written informed consent was obtained for all participants.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Huang, H., Zhou, H., Wang, W. et al. Prediction of acute kidney injury, sepsis and mortality in children with urinary CXCL10. Pediatr Res 92, 541–548 (2022). https://doi.org/10.1038/s41390-021-01813-y
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41390-021-01813-y
This article is cited by
-
Astrocyte-Derived CXCL10 Induces Neuronal Tau Hyperphosphorylation and Cognitive Impairments in Sepsis
Neuroscience Bulletin (2025)
-
Derivation and validation of urinary TIMP-1 for the prediction of acute kidney injury and mortality in critically ill children
Journal of Translational Medicine (2022)