Abstract
Background
Our understanding of the normative concentrations of urine biomarkers in premature neonates is limited.
Methods
We evaluated urine from 750 extremely low gestational age (GA) neonates without severe acute kidney injury (AKI) to determine how GA affects ten different urine biomarkers at birth and over the first 30 postnatal days. Then, we investigated if the urine biomarkers changed over time at 27, 30, and 34 weeks postmenstrual age (PMA). Next, we evaluated the impact of sex on urine biomarker concentrations at birth and over time. Finally, we evaluated if urine biomarkers were impacted by treatment with erythropoietin (Epo).
Results
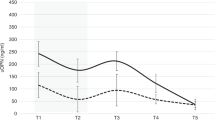
We found that all ten biomarker concentrations differ at birth by GA and that some urine biomarker concentrations increase, while others decrease over time. At 27 weeks PMA, 7/10 urine biomarkers differed by GA. By 30 weeks PMA, 5/10 differed, and by 34 weeks PMA, only osteopontin differed by GA. About half of the biomarker concentrations differed by sex, and 4/10 showed different rates of change over time between males vs. females. We found no differences in urine biomarkers by treatment group.
Conclusions
The temporal patterns, GA, and sex differences need to be considered in urine AKI biomarker analyses.
Impact
-
Urine biomarker concentrations differ by GA at birth.
-
Some urine biomarkers increase, while others decrease, over the first 30 postnatal days.
-
Most urine biomarkers differ by GA at 27 weeks PMA, but are similar by 34 weeks PMA.
-
Some urine biomarkers vary by sex in premature neonates.
-
Urine biomarkers did not differ between neonates randomized to placebo vs. Epo.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Change history
13 December 2021
A Correction to this paper has been published: https://doi.org/10.1038/s41390-021-01898-5
References
Jetton, J. G. et al. Incidence and outcomes of neonatal acute kidney injury (awaken): a multicentre, multinational, observational cohort study. Lancet Child Adolesc. Health 1, 184–194 (2017).
Koralkar, R. et al. Acute kidney injury reduces survival in very low birth weight infants. Pediatr. Res. 69, 354–358 (2011).
Askenazi, D. J., Griffin, R., McGwin, G., Carlo, W. & Ambalavanan, N. Acute kidney injury is independently associated with mortality in very low birthweight infants: a matched case-control analysis. Pediatr. Nephrol. 24, 991–997 (2009).
Askenazi, D. J. et al. Prevalence of acute kidney injury (Aki) in extremely low gestational age neonates (Elgan). Pediatr. Nephrol. 35, 1737–1748 (2020).
Goldstein, S. L. Acute kidney injury in children: prevention, treatment and rehabilitation. Contrib. Nephrol. 174, 163–172 (2011).
Soni, S. S., Ronco, C., Katz, N. & Cruz, D. N. Early diagnosis of acute kidney injury: the promise of novel biomarkers. Blood Purif. 28, 165–174 (2009).
Ostermann, M., Philips, B. J. & Forni, L. G. Clinical review: biomarkers of acute kidney injury: Where are we now? Crit. Care 16, 233 (2012).
Askenazi, D. J. et al. Baseline values of candidate urine acute kidney injury (Aki) biomarkers vary by gestational age in premature infants. Pediatr. Res. 70, 302–306 (2011).
Ahn, Y. H., Lee, J., Chun, J., Jun, Y. H. & Sung, T. J. Urine biomarkers for monitoring acute kidney injury in premature infants. Kidney Res. Clin. Pract. 39, 284–294 (2020).
Kamianowska, M., Szczepanski, M. & Wasilewska, A. Tubular and glomerular biomarkers of acute kidney injury in newborns. Curr. Drug Metab. 20, 332–349 (2019).
Sellmer, A. et al. Urinary neutrophil gelatinase-associated lipocalin in the evaluation of patent ductus arteriosus and aki in very preterm neonates: a cohort study. BMC Pediatr. 17, 7 (2017).
Tanigasalam, V., Bhat, B. V., Adhisivam, B., Sridhar, M. G. & Harichandrakumar, K. T. Predicting severity of acute kidney injury in term neonates with perinatal asphyxia using urinary neutrophil gelatinase associated lipocalin. Indian J. Pediatr. 83, 1374–1378 (2016).
Hanna, M. et al. Early urinary biomarkers of acute kidney injury in preterm infants. Pediatr. Res. 80, 218–223 (2016).
Chen, C. N. et al. Urinary neutrophil gelatinase-associated lipocalin levels in neonates. Pediatr. Neonatol. 57, 207–212 (2016).
Askenazi, D. J. et al. Acute kidney injury urine biomarkers in very low-birth-weight infants. Clin. J. Am. Soc. Nephrol. 11, 1527–1535 (2016).
Askenazi, D. J. et al. Urine biomarkers predict acute kidney injury in newborns. J. Pediatr. 161, 270–275 e271 (2012).
Askenazi, D. J. et al. Urine biomarkers predict acute kidney injury and mortality in very low birth weight infants. J. Pediatr. 159, 907–912.e901 (2011).
Balena-Borneman, J. et al. Biomarkers associated with bronchopulmonary dysplasia/mortality in premature infants. Pediatr. Res. 81, 519–525 (2017).
Shima, Y., Kumasaka, S. & Nishimaki, S. Urinary beta2-microglobulin and bronchopulmonary dysplasia: trends in preterm infants. Pediatr. Int. 59, 1169–1173 (2017).
Askenazi, D. J. et al. Baseline values of candidate urine acute kidney injury biomarkers vary by gestational age in premature infants. Pediatr. Res. 70, 302–306 (2011).
Saeidi, B. et al. Impact of gestational age, sex, and postnatal age on urine biomarkers in premature neonates. Pediatr. Nephrol. 30, 2037–2044 (2015).
Lavery, A. P. et al. Urinary Ngal in premature infants. Pediatr. Res. 64, 423–428 (2008).
Huynh, T. K. et al. Reference values of urinary neutrophil gelatinase-associated lipocalin in very low birth weight infants. Pediatr. Res. 66, 528–532 (2009).
Bennett, M. R., Nehus, E., Haffner, C., Ma, Q. & Devarajan, P. Pediatric reference ranges for acute kidney injury biomarkers. Pediatr. Nephrol. 30, 677–685 (2015).
Weber, A., Harrison, T. M., Sinnott, L., Shoben, A. & Steward, D. Plasma and urinary oxytocin trajectories in extremely premature infants during NICU hospitalization. Biol. Res. Nurs. 19, 549–558 (2017).
Nakata, Y., Okada, H., Itoh, S. & Kusaka, T. Developmental changes in urinary coproporphyrin ratio in premature infants. Pediatr. Int. 62, 65–69 (2020).
Juul, S. E., Mayock, D. E., Comstock, B. A. & Heagerty, P. J. Neuroprotective potential of erythropoietin in neonates; design of a randomized trial. Matern. Health Neonatol. Perinatol. 1, 27 (2015).
Juul, S. E. et al. A randomized trial of erythropoietin for neuroprotection in preterm infants. N. Engl. J. Med. 382, 233–243 (2020).
Kellum, J. A., Lameire, N. & Group, K. A. G. W. Diagnosis, evaluation, and management of acute kidney injury: a Kdigo summary (Part 1). Crit. Care 17, 204 (2013).
Thayyil, S., Sheik, S., Kempley, S. T. & Sinha, A. A gestation- and postnatal age-based reference chart for assessing renal function in extremely premature infants. J. Perinatol. 28, 226–229 (2008).
Bateman, D. A. et al. Serum creatinine concentration in very-low-birth-weight infants from birth to 34-36 wk postmenstrual age. Pediatr. Res. 77, 696–702 (2015).
Engle, W. A. American Academy of Pediatrics Committee on, F. & Newborn. Age terminology during the perinatal period. Pediatrics 114, 1362–1364 (2004).
Amaral Pedroso, L. et al. Acute kidney injury biomarkers in the critically ill. Clin. Chim. Acta 508, 170–178 (2020).
Devarajan, P. The current state of the art in acute kidney injury. Front. Pediatr. 8, 70 (2020).
Zeger, S. L. & Liang, K. Y. Longitudinal data analysis for discrete and continuous outcomes. Biometrics 42, 121–130 (1986).
Hinchliffe, S. A., Sargent, P. H., Howard, C. V., Chan, Y. F. & van Velzen, D. Human intrauterine renal growth expressed in absolute number of glomeruli assessed by the disector method and cavalieri principle. Lab. Invest. 64, 777–784 (1991).
Boohaker, L. et al. Absorbent materials to collect urine can affect proteomics and metabolomic biomarker concentrations. Clin. Chem. Lab. Med. 57, e134–e137 (2019).
Acknowledgements
We would like to thank Lynn Dill, RN and Emily Pao for their assistance in coordinating the REPaIReD study, and to Dana Pass for the preparation of the manuscript. We would like to thank the additional primary investigators, co-investigators, clinicians, research personnel, study team, and families who participated in the PENUT study. Some data presented in this study were previously presented as an e-poster abstract presentation at the 2020 American Society of Nephrology meeting.
Funding
Recombinant Erythropoietin for Protection of Infant Renal Disease (REPaIReD) Study is a NIHNIDDK-funded (R01 DK103608) ancillary study designed to look at kidney outcome in patients enrolled in the Preterm Erythropoietin Neuroprotection Trial (PENUT trial), which is a NIHNINDS-funded (U01 NS077953, U01 NS077955) trial. Urine creatinine was run at the UAB AKIO’Brien Center core (NIH P30-DK079337). The clinicaltrials.gov identifier is NCT01378273. Funding sources for this study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Author information
Authors and Affiliations
Consortia
Contributions
D.J.A. contributed to the conceptualization and design of the study, data analysis, and drafted the initial manuscript. B.A.H., R.H.S., P.J.H., P.B., S.E.J., S.L.G., and S.H. contributed to the conceptualization and design of the study, data analysis, and assisted in the manuscript preparation. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
All authors declare no real or perceived conflicts of interest that could affect the study design, collection, analyses, and interpretation of data, writing of the report, or the decision to submit for publication. For full disclosure, we provide here an additional list of other authors’ commitments and funding sources that are not directly related to this study: D.J.A. is a consultant for Baxter, Nuwellis, Medtronic, Bioporto, AKI foundation, and SeaStar. He also receives external education and research funding not related to this project from Baxter, Nuwelis, and Medtronic. S.L.G. reports personal fees from and a position as a consultant to Nuwellis, Renibus, ExThera, Reata, and Medtronic Inc. S.L.G. receives grant funding from and serves as a consultant and on a Speaker’s Bureau for Baxter Healthcare, Inc. S.L.G. receives grant funding and serves as a consultant for BioPorto, Inc. S.L.G. serves on a Speaker’s Bureau for Fresenius Medical Corporation. S.E.J. receives grant funding from NINDS and NICHD for studies not related to this project. P.J.H. and R.H.S. receive grant funding from NHLBI and PCORI for studies not related to this project.
Ethics committee approval
The University of Washington Institutional Review Board (IRB) approved this collaborative study, and each center received approval from its respective IRBs.
Consent statement
Parental/guardian consent was required for participation.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: The list of authors has been corrected and Brian Halloran has been added to the PDF.
Rights and permissions
About this article
Cite this article
Askenazi, D.J., Halloran, B.A., Heagerty, P.J. et al. Gestational age, sex, and time affect urine biomarker concentrations in extremely low gestational age neonates. Pediatr Res 92, 151–167 (2022). https://doi.org/10.1038/s41390-021-01814-x
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41390-021-01814-x
This article is cited by
-
Reducing NICU ventilator days by preventing fluid overload with the CAN-U-P-LOTS standardized bundle
Pediatric Research (2025)
-
Advocating for the inclusion of kidney health outcomes in neonatal research: best practice recommendations by the Neonatal Kidney Collaborative
Journal of Perinatology (2024)
-
Neonatal hyperoxia exposure leads to developmental programming of cardiovascular and renal disease in adult rats
Scientific Reports (2024)
-
Urine acute kidney injury biomarkers in extremely low gestational age neonates: a nested case control study of 21 candidate urine biomarkers
Pediatric Nephrology (2023)