Abstract
Background
Hypothermia is widely used for infants with hypoxic–ischemic neonatal encephalopathy but its impact remains poorly described at a population level. We aimed to describe brain imaging in infants born at ≥36 weeks’ gestation, with moderate/severe encephalopathy treated with hypothermia.
Methods
Descriptive analysis of brain MRI and discharge neurological examination for infants included in the French national multicentric prospective observational cohort LyTONEPAL.
Results
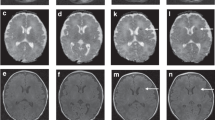
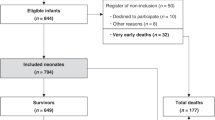
Among 575 eligible infants, 479 (83.3%) with MRI before 12 days of life were included. MRI was normal for 48.2% (95% CI 43.7–52.8). Among infants with brain injuries, 62.5% (95% CI 56.2–68.5) had damage to more than one structure, 19.8% (95% CI 15.0–25.3) showed a pattern-associating injuries of basal ganglia/thalami (BGT), white matter (WM) and cortex. Overall, 68.4% (95% CI 62.0–74.3) of infants with normal MRI survived with a normal neurological examination. The rate of death was 15.4% (95% CI 12.3–19.0), predominantly for infants with the combined BGT, cortex, and/or WM injuries.
Conclusions
Among infants with neonatal encephalopathy treated with hypothermia, two-thirds of those with normal MRI survived with a normal neurological examination at discharge. When present, brain injuries often involved more than one structure.
Trial registration
The trial was registered at ClinicalTrials.gov (NCT02676063).
Impact
-
In this multicentric cohort of infants with neonatal encephalopathy (LYTONEPAL) two-thirds survived with normal MRI and neurological examination at discharge.
-
In total, 10% of newborns showed a pattern associating injuries of the basal ganglia—thalami, white matter, and cortex, which was correlated with a high risk of death at discharge.
-
The evolution of MRI techniques and sequences in the era of hypothermia calls for a revisiting of imaging protocol in neonatal encephalopathy, especially for the timing.
-
The neurological examination did not give evidence of brain injuries, thus questioning the reproducibility of the clinical exam or the neonatal brain functionality.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Pierrat, V. et al. Prevalence, causes, and outcome at 2 years of age of newborn encephalopathy: population based study. Arch. Dis. Child. Fetal Neonatal Ed. 90, F257–F261 (2005).
Kurinczuk, J. J., White-Koning, M. & Badawi, N. Epidemiology of neonatal encephalopathy and hypoxic-ischaemic encephalopathy. Early Hum. Dev. 86, 329–338 (2010).
Weeke, L. C. et al. A novel magnetic resonance imaging score predicts neurodevelopmental outcome after perinatal asphyxia and therapeutic hypothermia. J. Pediatr. 192, 33–40.e2 (2018).
Bednarek, N. et al. Impact of therapeutic hypothermia on MRI diffusion changes in neonatal encephalopathy. Neurology 78, 1420–1427 (2012).
Okereafor, A. et al. Patterns of brain injury in neonates exposed to perinatal sentinel events. Pediatrics 121, 906–914 (2008).
van Kooij, B. J. M. et al. Serial MRI and neurodevelopmental outcome in 9- to 10-year-old children with neonatal encephalopathy. J. Pediatr. 157, 221–227.e2 (2010).
Martinez-Biarge, M. et al. White matter and cortical injury in hypoxic-ischemic encephalopathy: antecedent factors and 2-year outcome. J. Pediatr. 161, 799–807 (2012).
Rutherford, M. et al. Assessment of brain tissue injury after moderate hypothermia in neonates with hypoxic–ischaemic encephalopathy: a nested substudy of a randomised controlled trial. Lancet Neurol. 9, 39–45 (2010).
Inder, T. E. et al. Randomized trial of systemic hypothermia selectively protects the cortex on MRI in term hypoxic-ischemic encephalopathy. J. Pediatr. 145, 835–837 (2004).
Miller, S. P. et al. Patterns of brain injury in term neonatal encephalopathy. J. Pediatr. 146, 453–460 (2005).
Rao, S. et al. Long-term outcomes and risk factors associated with acute encephalitis in children. J. Pediatr. Infect. Dis. Soc. https://doi.org/10.1093/jpids/piv075 (2015).
Barnett, A. et al. Neurological and perceptual-motor outcome at 5 - 6 years of age in children with neonatal encephalopathy: relationship with neonatal brain MRI. Neuropediatrics 33, 242–248 (2002).
Kasdorf, E., Engel, M., Heier, L. & Perlman, J. M. Therapeutic hypothermia in neonates and selective hippocampal injury on diffusion-weighted magnetic resonance imaging. Pediatr. Neurol. 51, 104–108 (2014).
Kuenzle, C. et al. Prognostic value of early MR imaging in term infants with severe perinatal asphyxia. Neuropediatrics 25, 191–200 (1994).
Walsh, B. H. et al. The frequency and severity of magnetic resonance imaging abnormalities in infants with mild neonatal encephalopathy. J. Pediatr. 187, 26–33.e1 (2017).
Jacobs, S. E. et al. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst. Rev. CD003311. https://doi.org/10.1002/14651858.CD003311.pub3 (2013).
Saliba, E. & Debillon, T. Hypothermia for hypoxic-ischemic encephalopathy in fullterm newborns. Arch. Pediatr. Organe . Soc. Francaise Pediatr. 17(Suppl 3), S67–S77 (2010).
Debillon, T., Bednarek, N. & Ego, A. LyTONEPAL: long term outcome of neonatal hypoxic encephalopathy in the era of neuroprotective treatment with hypothermia: a French population-based cohort. BMC Pediatr. 18, 255 (2018).
Sarnat, H. B. & Sarnat, M. S. Neonatal encephalopathy following fetal distress. A clinical and electroencephalographic study. Arch. Neurol. 33, 696–705 (1976).
Mercuri, E. et al. Head growth in infants with hypoxic-ischemic encephalopathy: correlation with neonatal magnetic resonance imaging. Pediatrics 106, 235–243 (2000).
D’Alton, M. E. et al. Executive summary: Neonatal encephalopathy and neurologic outcome, second edition. Report of the American College of Obstetricians and Gynecologists’ Task Force on Neonatal Encephalopathy. Obstet. Gynecol. 123, 896–901 (2014).
Agut, T. et al. Early identification of brain injury in infants with hypoxic ischemic encephalopathy at high risk for severe impairments: accuracy of MRI performed in the first days of life. BMC Pediatr. 14, 177 (2014).
Charon, V. et al. Comparison of early and late MRI in neonatal hypoxic-ischemic encephalopathy using three assessment methods. Pediatr. Radiol. 45, 1988–2000 (2015).
Charon, V. et al. Early MRI in neonatal hypoxic-ischaemic encephalopathy treated with hypothermia: prognostic role at 2-year follow-up. Eur. J. Radiol. 85, 1366–1374 (2016).
Barkovich, A. J. et al. Prediction of neuromotor outcome in perinatal asphyxia: evaluation of MR scoring systems. AJNR 19, 143–149 (1998).
Azzopardi, D. et al. The TOBY Study. Whole body hypothermia for the treatment of perinatal asphyxial encephalopathy: a randomised controlled trial. BMC Pediatr. 8, 17 (2008).
Shankaran, S. et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N. Engl. J. Med 353, 1574–1584 (2005).
Shankaran, S. et al. Neonatal magnetic resonance imaging pattern of brain injury as a biomarker of childhood outcomes following a trial of hypothermia for neonatal hypoxic-ischemic encephalopathy. J. Pediatr. 167, 987–993.e3 (2015).
Guillot, M. et al. Influence of timing of initiation of therapeutic hypothermia on brain MRI and neurodevelopment at 18 months in infants with HIE: a retrospective cohort study. BMJ Paediatr. Open 3, e000442 (2019).
Shankaran, S. et al. Childhood outcomes after hypothermia for neonatal encephalopathy. N. Engl. J. Med. 366, 2085–2092 (2012).
Shapiro, K. A. et al. Early changes in brain structure correlate with language outcomes in children with neonatal encephalopathy. NeuroImage Clin. 15, 572–580 (2017).
Tusor, N. et al. Prediction of neurodevelopmental outcome after hypoxic-ischemic encephalopathy treated with hypothermia by diffusion tensor imaging analyzed using tract-based spatial statistics. Pediatr. Res. 72, 63–69 (2012).
Cheong, J. L. Y. et al. Prognostic utility of magnetic resonance imaging in neonatal hypoxic-ischemic encephalopathy: substudy of a randomized trial. Arch. Pediatr. Adolesc. Med. 166, 634–640 (2012).
De Wispelaere, L. A. et al. Electroencephalography and brain magnetic resonance imaging in asphyxia comparing cooled and non-cooled infants. Eur. J. Paediatr. Neurol. 23, 181–190 (2019).
Sargent, M. A., Poskitt, K. J., Roland, E. H., Hill, A. & Hendson, G. Cerebellar vermian atrophy after neonatal hypoxic-ischemic encephalopathy. AJNR 25, 1008–1015 (2004).
Le Strange, E., Saeed, N., Cowan, F. M., Edwards, A. D. & Rutherford, M. A. MR imaging quantification of cerebellar growth following hypoxic-ischemic injury to the neonatal brain. AJNR 25, 463–468 (2004).
Annink, K. V. et al. Cerebellar injury in term neonates with hypoxic-ischemic encephalopathy is underestimated. Pediatr. Res. https://doi.org/10.1038/s41390-020-01173-z (2020).
Murray, D. M. et al. The predictive value of early neurological examination in neonatal hypoxic-ischaemic encephalopathy and neurodevelopmental outcome at 24 months. Dev. Med. Child Neurol. 52, e55–e59 (2010).
Grass, B. et al. Short-term neurological improvement in neonates with hypoxic-ischemic encephalopathy predicts neurodevelopmental outcome at 18–24 months. J. Perinat. Med. 48, 296–303 (2020).
Brazelton, T. B. The Brazelton Neonatal Behavior Assessment Scale: introduction. Monogr. Soc. Res. Child Dev. 43, 1–13 (1978).
Sánchez Fernández, I., Morales-Quezada, J. L., Law, S. & Kim, P. Prognostic value of brain magnetic resonance imaging in neonatal hypoxic-ischemic encephalopathy: a meta-analysis. J. Child Neurol. 32, 1065–1073 (2017).
Shankaran, S. et al. Brain injury following trial of hypothermia for neonatal hypoxic-ischaemic encephalopathy. Arch. Dis. Child. Fetal Neonatal Ed. 97, F398–F404 (2012).
Acknowledgements
We thank members of the LyTONEPAL Study Group and all the regional teams participating in the study and all French maternity and neonatal units for their substantial contribution to the acquisition of data; Laura Smales and Grace Stockton for their helpful contribution to proofreading. We are grateful for the participation of all families of infants in the LyTONEPAL cohort study. We thank the American Memorial Committee for their considerable support. This study was funded by the National Program for Clinical Research (PHRC-N-13-0327).
The LyTONEPAL Study Group Collaborators
Auvergne Rhone Alpes: N. Bouchon-Guedj (Chambéry), G. Remerand (Clermont-Ferrand), M. Chevallier (Grenoble), O. Claris (Lyon, HFME), C.M. Loys (Lyon, Croix Rousse), H. Patural (Saint-Etienne), Bourgogne Franche Comté: T. Dabudyk (Besançon), C. Chantegret (Dijon), Bretagne: J.M. Roué (Brest), M. Gromand (Rennes), A. Busnel (St-Brieuc), A. Sevestre (Vannes), Centre Val de Loire: J. Guerreiro (Orléans), G. Favrais (Tours), Grand Est: J. Nakhleh (Mulhouse), N. Bednarek (Reims), D. Astruc, (Strasbourg), B. Kassis-Makhoul (Troyes), Hauts de France: G. Ghostine (Amiens), J. Ghesquiere (Arras), L. Egreteau (Calais), S.M. Dhahbi (Creil), S. Klosowski (Lens), F. Flamein (Lille), J. Balitalike (Valenciennes), Ile-de-France: D. Brau (Argenteuil), V. Zupan-Simunek (Clamart), C. Huon (Colombes), M. Tauzin (Créteil), M. Merhi (Evry), N. Le Sache (Le Kremlin-Bicêtre), B. Heller Roussin (Montreuil), D. Mellah (Meaux), A. Lapillonne, E. Leroy Terquem (Paris, Necker), J. Patkai (Paris, Port Royal), V. Biran (Paris, Robert Debré) I. Guellec (Paris, Trousseau), A. Durandy (Poissy), P. Boize (Pontoise), F. Goudjil (St Denis), Nouvelle Aquitaine: P. Jouvencel (Bayonne), O. Brissaud (Bordeaux), F. Mons (Limoges), K. Norbert (Pau), A. Parizel (Poitiers), Occitanie: G. Cambonie (Montpellier), M. Di Maio (Nîmes), R. Salloum (Perpignan), M.O. Marcoux (Toulouse), Pays de Loire: S. Le Bouedec (Angers), C. Flamant (Nantes), Y. Montcho (Le Mans), Provence Alpes Côte d’Azur: C. Desrobert (Marseille La Conception), V. Brevaut-Malaty (Marseille, Nord), F. Casagrande (Nice), R. Salloum (Perpignan), Martinique: S. Ketterer Martinon (Fort de France), Normandie: A. Cénéric (Caen), J. Mourdie (Le Havre), A. Chadie (Rouen), La Réunion: M. Carbonnier (Saint-Pierre), D. Ramful (Saint-Denis).
Author information
Authors and Affiliations
Contributions
J.B., T.D., A.V., C.L., A.E., and P.-Y.A. had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. J.B., T.D., N.B., V.P., A.E., and P.-Y.A. conceptualized the study and wrote the manuscript. J.B. and A.V. performed the statistical analysis. C.L., A.V., M.A., and L.-H.P. coordinated data collection and had responsibility for technical support. T.D. obtained funding and supervised the study. All authors contributed to the data analysis and interpretation of the results and reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Consent statement
Informed consent was obtained from both parents. The study protocol was approved by the Advisory National Committee on the treatment of personal health data for research purposes (Comité Consultatif sur le Traitement de l’Information en matière de Recherche sur la Santé, reference no. 14.724). Authorizations were obtained from the National French data protection authority (Commission Nationale Informatique et Libertés, DR-2015-136) and the regional ethics committee (Comité de Protection des Personnes Sud Est; Institutional Review Board no. 5891).
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Beck, J., Bednarek, N., Pierrat, V. et al. Cerebral injuries in neonatal encephalopathy treated with hypothermia: French LyTONEPAL cohort. Pediatr Res 92, 880–887 (2022). https://doi.org/10.1038/s41390-021-01846-3
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41390-021-01846-3