Abstract
Background
Preterm infants are generally fed through nasogastric enteral feeding tubes (NEFTs). The aim of this work was to evaluate the role of NEFTs in the initial colonization of the preterm gut and its evolution within the first 2 weeks after birth.
Methods
For this purpose, fecal and NEFT-derived samples from 30 preterm infants hospitalized in a neonatal intensive care unit (NICU) were collected from birth to the second week of life. Samples were cultivated in ten culture media, including three for the isolation of antibiotic-resistant microorganisms.
Results
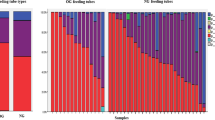
Isolates (561) were identified by 16S ribosomal RNA gene sequencing. Although the first NEFTs inserted into the neonates after birth were rarely colonized, analysis of NEFTs and fecal samples over time revealed a significant increase in bacterial abundance, diversity, and detection frequency. Results showed a parallel colonization between time-matched NEFTs and fecal samples, suggesting an ongoing bidirectional transfer of bacteria from the neonatal gut to the NEFTs and vice versa.
Conclusions
In short-term hospitalization, length is by far the determinant factor for the early colonization of preterm infants. As NEFT populations reflect the bacterial populations that are colonizing the preterm in a precise moment, their knowledge could be useful to prevent the dissemination of antibiotic-resistant strains.
Impact
-
The hospital environment modulates preterm colonization immediately after birth.
-
The colonization of preterm feces and NEFTs occurs in parallel.
-
There is an ongoing bidirectional transfer of microorganisms from the neonatal gut to the NEFTs and vice versa.
-
Bacterial communities inside NEFTs could act as reservoirs of antibiotic resistance genes.
-
NEFT populations reflect the bacteria that are colonizing the preterm at a precise moment.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
WHO. Preterm birth. https://www.who.int/en/news-room/fact-sheets/detail/preterm-birth (2018).
Howson, C. P., Kinney, M. V., McDougall, L. & Lawn, J. E. Born too soon: preterm birth matters. Reprod. Health 10, 1–9 (2013).
Moles, L. et al. Serratia marcescens colonization in preterm neonates during their neonatal intensive care unit stay. Antimicrob. Resist. Infect. Control 8, 1–8 (2019).
Arboleya, S. et al. Establishment and development of intestinal microbiota in preterm neonates. FEMS Microbiol. Ecol. 79, 763–772 (2012).
Vinall, J. & Grunau, R. E. Impact of repeated procedural pain-related stress in infants born very preterm. Pediatr. Res. 75, 584–587 (2014).
Patel, K., Konduru, K., Patra, A. K., Chandel, D. S. & Panigrahi, P. Trends and determinants of gastric bacterial colonization of preterm neonates in a NICU setting. PLoS ONE 10, e0114664 (2015).
Henderickx, J. et al. The preterm gut microbiota: an inconspicuous challenge in nutritional neonatal care. Front. Cell Infect. Microbiol. 9, 85 (2019).
Jia, J. et al. Impact of postnatal antibiotics and parenteral nutrition on the gut microbiota in preterm infants during early life. J. Parent. Enter. Nutr. 44, 639–654 (2020).
Bu’Lock, F., Woolridge, M. W. & Baum, J. D. Development of co-ordination of sucking, swallowing and breathing: ultrasound study of term and preterm infants. Dev. Med. Child Neurol. 32, 669–678 (1990).
Petersen, S. M., Greisen, G. & Krogfelt, K. A. Nasogastric feeding tubes from a neonatal department yield high concentrations of potentially pathogenic bacteria- even 1 d after insertion. Pediatr. Res. 80, 395–400 (2016).
Berthelot, P. et al. Nosocomial colonization of premature babies with Klebsiella oxytoca: probable role of enteral feeding procedure in transmission and control of the outbreak with the use of gloves. Infect. Control Hosp. Epidemiol. 22, 148–151 (2001).
Martineau, C. et al. Serratia marcescens outbreak in a neonatal intensive care unit: new insights from next-generation sequencing applications. J. Clin. Microbiol. 56, e00235–18 (2018).
Young, B. E. et al. Epidemiologic features and clinical course of patients infected with SARS-CoV-2 in Singapore. JAMA 323, 1488–1494 (2020).
Schwiertz, A. et al. Development of the intestinal bacterial composition in hospitalized preterm infants in comparison with breast-fed, full-term infants. Pediatr. Res. 54, 393–399 (2003).
Brooks, B. et al. Microbes in the neonatal intensive care unit resemble those found in the gut of premature infants. Microbiome 2, 1 (2014).
Gómez, M. et al. Early gut colonization of preterm infants: effect of enteral feeding tubes. J. Pediatr. Gastroenterol. Nutr. 62, 893–900 (2016).
Ogrodzki, P. et al. Rapid in situ imaging and whole genome sequencing of biofilm in neonatal feeding tubes: a clinical proof of concept. Sci. Rep. 7, 15948 (2017).
Donlan, R. M. Biofilms and device-associated infections. Emerg. Infect. Dis. 7, 277 (2001).
Mehall, J. R., Kite, C. A., Gilliam, C. H., Jackson, R. J. & Smith, S. D. Enteral feeding tubes are a reservoir for nosocomial antibiotic-resistant pathogens. J. Pediatr. Surg. 37, 1011–1012 (2002).
ECDC (European Centre for Disease Prevention and Control). Healthcare-associated infections acquired in intensive care units. ECDC. Annual epidemiological report for 2016. Stockholm: ECDC; 2018.
Hagbø, M. et al. Experimental support for multidrug resistance transfer potential in the preterm infant gut microbiota. Pediatr. Res. 88, 57–65 (2020).
Baldassarre, M. et al. Dysbiosis and prematurity: Is there a role for probiotics? Nutrients 11, 1273 (2019).
Høiby, N. et al. ESCMID guideline for the diagnosis and treatment of biofilm infections 2014. Clin. Microbiol. Infect. 21, S1–S25 (2015).
Zingg, W. et al. Health-care-associated infections in neonates, children, and adolescents: an analysis of paediatric data from the European Centre for Disease Prevention and Control point-prevalence survey. Lancet Infect. Dis. 17, 381–389 (2017).
Hurrell, E. et al. Neonatal enteral feeding tubes as loci for colonisation by members of the Enterobacteriaceae. BMC Infect. Dis. 9, 146 (2009).
Poets, C. F. Gastroesophageal reflux: a critical review of its role in preterm infants. Pediatrics 113, e128–e132 (2004).
Segal, R., Pogoreliuk, I., Dan, M., Baumoehl, Y. & Leibovitz, A. Gastric microbiota in elderly patients fed via nasogastric tubes for prolonged periods. J. Hosp. Infect. 63, 79–83 (2006).
Mehall, J. R. et al. Prospective study of the incidence and complications of bacterial contamination of enteral feeding in neonates. J. Pediatr. Surg. 37, 1177–1182 (2002). (b).
Kim, H., Ryu, J. H. & Beuchat, L. R. Attachment of and biofilm formation by Enterobacter sakazakii on stainless steel and enteral feeding tubes. Appl. Environ. Microbiol. 72, 5846–5856 (2006).
Cong, X. et al. Gut microbiome developmental patterns in early life of preterm infants: impacts of feeding and gender. PLoS ONE 11, e0152751 (2016).
Mediano, P. et al. Microbial diversity in milk of women with mastitis: potential role of coagulase-negative Staphylococci, Viridans group Streptococci, and Corynebacteria. J. Hum. Lact. 33, 309–318 (2017).
Pohlert, T. PMCMRplus: calculate pairwise multiple comparisons of mean rank sums extended. R package version 1.9.0. https://CRAN.R-project.org/package=PMCMRplus (2021).
Hervé, M. RVAideMemoire: testing and plotting procedures for biostatistics. R package version 0.9-80. https://CRAN.R-project.org/package=RVAideMemoire (2021).
Mangiafico, S. rcompanion: functions to support extension education program evaluation. R package version 2.4.1. https://CRAN.R-project.org/package=rcompanion (2021).
Warnes, G. R. et al. gplots: various R programming tools for plotting data. R package version 3.1.1. https://CRAN.R-project.org/package=gplots (2020).
Oksanen, J. et al. Vegan: community ecology package. http://CRAN.R-project.org/package=vegan (2012).
Dahl, C. et al. Preterm infants have distinct microbiomes not explained by mode of delivery, breastfeeding duration or antibiotic exposure. Int. J. Epidemiol. 47, 1658–1669 (2018).
Gasparrini, A. J. et al. Antibiotic perturbation of the preterm infant gut microbiome and resistome. Gut Microbes 7, 443–449 (2016).
Liu, W. Y., Wong, C. F., Chung, K. M., Jiang, J. W. & Leung, F. C. Comparative genome analysis of Enterobacter cloacae. PLoS ONE 8, e74487 (2013).
Bussy, V., Marechal, F. & Nasca, S. Microbial contamination of enteral feeding tubes occurring during nutritional treatment. J. Parenter. Enter. Nutr. 16, 552–557 (1992).
Liu, R. et al. Molecular analysis of long-term biofilm formation on PVC and cast iron surfaces in drinking water distribution system. J. Environ. Sci. 26, 865–874 (2014).
Bur, S., Preissner, K. T., Herrmann, M. & Bischoff, M. The Staphylococcus aureus extracellular adherence protein promotes bacterial internalization by keratinocytes independent of fibronectin-binding proteins. J. Invest. Dermatol. 133, 2004–2012 (2013).
Puga, C. H., Dahdouh, E., SanJose, C. & Orgaz, B. Listeria monocytogenes colonizes Pseudomonas fluorescens biofilms and induces matrix over-production. Front. Microbiol. 9, 1706 (2018).
Jara, J. et al. Role of Lactobacillus biofilms in Listeria monocytogenes adhesion to glass surfaces. Int. J. Food Microbiol. 334, 108804 (2020).
Donnet-Hughes, A. et al. Potential role of the intestinal microbiota of the mother in neonatal immune education. Proc. Nutr. Soc. 69, 407–415 (2010).
Groer, M. W. et al. Development of the preterm infant gut microbiome: a research priority. Microbiome 2, 38 (2014).
Wang, Z. et al. Comparing gut microbiome in mothers’ own breast milk- and formula-fed moderate-late preterm infants. Front. Microbiol. 11, 891 (2020).
Jiménez, E. et al. Staphylococcus epidermidis: a differential trait of the fecal microbiota of breast-fed infants. BMC Microbiol. 8, 143 (2008).
Moles, L. et al. Bacterial diversity in meconium of preterm neonates and evolution of their fecal microbiota during the first month of life. PLoS ONE 8, e66986 (2013).
Swartz, M. N. Hospital-acquired infections: diseases with increasingly limited therapies. Proc. Natl Acad. Sci. USA 91, 2420–2427 (1994).
de Man, P. et al. Enterobacter species in a pediatric hospital: horizontal transfer or selection in individual patients? J. Infect. Dis. 184, 211–214 (2001).
Gregory, K. E. et al. Influence of maternal breast milk ingestion on acquisition of the intestinal microbiome in preterm infants. Microbiome 4, 68 (2016).
Macpherson, A. J., de Agüero, M. G. & Ganal-Vonarburg, S. C. How nutrition and the maternal microbiota shape the neonatal immune system. Nat. Rev. Immunol. 17, 508–517 (2017).
Cong, X. et al. Influence of feeding type on gut microbiome development in hospitalized preterm infants. Nurs. Res. 66, 123–133 (2017).
Fernández, L., Ruiz, L., Jara, J., Orgaz, B. & Rodríguez, J. M. Strategies for the preservation, restoration and modulation of the human milk microbiota. implications for human milk banks and neonatal intensive care units. Front. Microbiol. 9, 2676 (2018).
Beghetti, I. et al. Human milk’s hidden gift: implications of the milk microbiome for preterm infants’ health. Nutrients 11, 2944 (2019).
Underwood, M. A. et al. A randomized placebo-controlled comparison of 2 prebiotic/probiotic combinations in preterm infants: impact on weight gain, intestinal microbiota, and fecal short-chain fatty acids. J. Pediatr. Gastroenterol. Nutr. 48, 216–225 (2009).
Esaiassen, E. et al. Effects of probiotic supplementation on the gut microbiota and antibiotic resistome development in preterm infants. Front. Pediatr. 6, 347 (2018).
Masuda, N. et al. Contribution of the MexX-MexY-OprM efflux system to intrinsic resistance in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 44, 2242–2246 (2000).
Jernberg, C., Löfmark, S., Edlund, C. & Jansson, J. K. Long-term ecological impacts of antibiotic administration on the human intestinal microbiota. ISME J. 1, 56–66 (2007).
Cox, L. M. et al. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell 158, 705–721 (2014).
Nobel, Y. R. et al. Metabolic and metagenomic outcomes from early-life pulsed antibiotic treatment. Nat. Commun. 6, 7486 (2015).
Flemming, H. C. & Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 8, 623–633 (2010).
Fux, C. A., Costerton, J. W., Stewart, P. S. & Stoodley, P. Survival strategies of infectious biofilms. Trends Microbiol. 13, 34–40 (2005).
Sabater, S. et al. Monitoring the effect of chemicals on biological communities. The biofilm as an interface. Anal. Bioanal. Chem. 387, 1425–1434 (2007).
Acknowledgements
We thank the nursing team of the Neonatology Unit of La Paz University Hospital in Madrid (Spain) for their assistance and the Spanish Ministry of Economy and Competitiveness and the Spanish Pediatric Association Grant (2015) for their funding.
Funding information
This work has been funded by the Spanish Ministry of Economy and Competitiveness (project ref. PID2019-105606RB-I00) and by the Spanish Pediatric Association Grant (AEP, 2015).
Author information
Authors and Affiliations
Contributions
J.J.P. and I.C.N carried out the microbiological analysis of the samples and wrote the first draft of the manuscript. C.A.R. and L.F.A carried out the statistical analysis. B.M.- S. coordinated the clinical part of the study. B.M.-S, B.C.J., E.E.P., and M.S.P. enrolled infants and collected the clinical data. M.S.P., B.O.M., and J.M.R. conceived and designed the study. L.F.A. contributed to the study design. All authors contributed to the analysis, and/or interpretation of data. All authors revised the article critically and approved the final version to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of La Paz University Hospital of Madrid, Spain (protocol code PI-3199 and date of approval, 27 June 2018). Parents or guardians provided written informed consent prior to enrollment.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jara Pérez, J., Moreno-Sanz, B., Castro Navarro, I. et al. Nasogastric enteral feeding tubes modulate preterm colonization in early life. Pediatr Res 92, 838–847 (2022). https://doi.org/10.1038/s41390-021-01852-5
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-021-01852-5