Abstract
Background
This study aimed to identify key microRNAs (miRNAs), pathways, and target genes mediating Hirschsprung’s disease (HSCR) pathogenesis and identify the diagnostic potential of miRNAs.
Methods
The Gene Expression Omnibus database and reverse transcription-quantitative PCR were used to compare miRNA expression between ganglionic and aganglionic colon tissues of children with HSCR, and the TAM 2.0 database was used to identify colon tissue-specific miRNAs. The StarBase database, TargetScan database, luciferase reporter, and western blot assays were used to analyze miRNA–messenger RNA interactions. OmicShare was used to perform functional and pathway enrichment analyses of the target genes. Migration assays were performed to validate the functions of the miRNAs.
Results
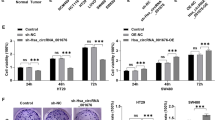
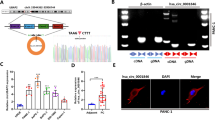
The TAM 2.0 database analysis and reverse transcription-quantitative PCR showed that hsa-miR-192-5p, hsa-miR-200a-3p, and hsa-miR-200b-3p were colon tissue-specific and upregulated in aganglionic colon tissue compared to paired ganglionic colon tissue. These three miRNAs effectively reduced cell viability and migration. Luciferase reporter and western blot assays verified the direct interaction between these three miRNAs and the target genes of ZEB2 and FNDC3B. Furthermore, the plasma levels of these miRNAs were higher in HSCR patients than in non-HSCR patients.
Conclusions
Three plasma miRNAs (hsa-miR-192-5p, hsa-miR-200a-3p, and hsa-miR-200b-3p) are potential peripheral HSCR biomarkers.
Impact
-
The molecular mechanisms underlying HSCR are unclear. HSCR is most accurately diagnosed using rectal biopsy samples, and no consensus has been reached on the use of blood-based tests for HSCR diagnosis. Circulating miRNAs may be candidate diagnostic HSCR biomarkers because they are typically easily detectable, stable, and tissue-specific. Three plasma miRNAs (miR-200a-3p, miR-192-5p, and miR-200b-3p) are potential peripheral HSCR biomarkers.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Burkardt, D. D., Graham, J. J., Short, S. S. & Frykman, P. K. Advances in Hirschsprung disease genetics and treatment strategies: an update for the primary care pediatrician. Clin. Pediatr. 53, 71–81 (2014).
Soret, R. et al. A collagen VI-dependent pathogenic mechanism for Hirschsprung’s disease. J. Clin. Invest. 125, 4483–4496 (2015).
Jaroy, E. G. et al. “Too much guts and not enough brains”: (epi)genetic mechanisms and future therapies of Hirschsprung disease—a review. Clin. Epigenet. 11, 135 (2019).
Lof, G. A. et al. Maternal risk factors and perinatal characteristics for Hirschsprung disease. Pediatrics 138, e20154608 (2016).
Bettolli, M. et al. Colonic dysmotility in postsurgical patients with Hirschsprung’s disease. Potential significance of abnormalities in the interstitial cells of Cajal and the enteric nervous system. J. Pediatr. Surg. 43, 1433–1438 (2008).
Jain, S. et al. Organotypic specificity of key RET adaptor-docking sites in the pathogenesis of neurocristopathies and renal malformations in mice. J. Clin. Invest. 120, 778–790 (2010).
Miyamoto, R. et al. Loss of Sprouty2 partially rescues renal hypoplasia and stomach hypoganglionosis but not intestinal aganglionosis in Ret Y1062F mutant mice. Dev. Biol. 349, 160–168 (2011).
Sanchez, M. P. et al. Renal agenesis and the absence of enteric neurons in mice lacking GDNF. Nature 382, 70–73 (1996).
Cantrell, V. A. et al. Interactions between Sox10 and EdnrB modulate penetrance and severity of aganglionosis in the Sox10Dom mouse model of Hirschsprung disease. Hum. Mol. Genet. 13, 2289–2301 (2004).
Stanchina, L., Van de Putte, T., Goossens, M., Huylebroeck, D. & Bondurand, N. Genetic interaction between Sox10 and Zfhx1b during enteric nervous system development. Dev. Biol. 341, 416–428 (2010).
Van de Putte, T. et al. Mice lacking ZFHX1B, the gene that codes for Smad-interacting protein-1, reveal a role for multiple neural crest cell defects in the etiology of Hirschsprung disease-mental retardation syndrome. Am. J. Hum. Genet. 72, 465–470 (2003).
Heuckeroth, R. O. Hirschsprung disease—integrating basic science and clinical medicine to improve outcomes. Nat. Rev. Gastroenterol. Hepatol. 15, 152–167 (2018).
Ambartsumyan, L., Smith, C. & Kapur, R. P. Diagnosis of Hirschsprung disease. Pediatr. Dev. Pathol. 23, 8–22 (2020).
Sergi, C. M., Caluseriu, O., McColl, H. & Eisenstat, D. D. Hirschsprung’s disease: clinical dysmorphology, genes, micro-RNAs, and future perspectives. Pediatr. Res. 81, 177–191 (2017).
Shen, Z. et al. Microarray expression profiling of dysregulated long non-coding RNAs in Hirschsprung’s disease reveals their potential role in molecular diagnosis. Neurogastroenterol. Motil. 28, 266–273 (2016).
Huntzinger, E. & Izaurralde, E. Gene silencing by microRNAs: contributions of translational repression and mRNA decay. Nat. Rev. Genet. 12, 99–110 (2011).
Bartel, D. P. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116, 281–297 (2004).
Maffioletti, E., Tardito, D., Gennarelli, M. & Bocchio-Chiavetto, L. Micro spies from the brain to the periphery: new clues from studies on microRNAs in neuropsychiatric disorders. Front. Cell. Neurosci. 8, 75 (2014).
Vishnoi, A. & Rani, S. MiRNA biogenesis and regulation of diseases: an overview. Methods Mol. Biol. 1509, 1–10 (2017).
Saliminejad, K., Khorram, K. H., Soleymani, F. S. & Ghaffari, S. H. An overview of microRNAs: biology, functions, therapeutics, and analysis methods. J. Cell. Physiol. 234, 5451–5465 (2019).
Wen, Z. et al. Circular RNA CCDC66 targets DCX to regulate cell proliferation and migration by sponging miR-488-3p in Hirschsprung’s disease. J. Cell. Physiol. 234, 10576–10587 (2019).
Tang, W. et al. Aberrant reduction of MiR-141 increased CD47/CUL3 in Hirschsprung’s disease. Cell. Physiol. Biochem. 32, 1655–1667 (2013).
Li, Y. et al. Long non-coding RNA FAL1 functions as a ceRNA to antagonize the effect of miR-637 on the down-regulation of AKT1 in Hirschsprung’s disease. Cell Prolif. 51, e12489 (2018).
Edgar, R., Domrachev, M. & Lash, A. E. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 30, 207–210 (2002).
Li, J. et al. TAM 2.0: tool for microRNA set analysis. Nucleic Acids Res. 46, W180–W185 (2018).
Li, J. H., Liu, S., Zhou, H., Qu, L. H. & Yang, J. H. starBase v2.0: decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA interaction networks from large-scale CLIP-Seq data. Nucleic Acids Res. 42, D92–D97 (2014).
Liu, J. A. et al. Identification of GLI mutations in patients with Hirschsprung disease that disrupt enteric nervous system development in mice. Gastroenterology 149, 1837–1848 (2015).
Olsen, R. R. et al. MYCN induces neuroblastoma in primary neural crest cells. Oncogene. 36, 5075–5082 (2017).
Anitha, M. et al. Characterization of fetal and postnatal enteric neuronal cell lines with improvement in intestinal neural function. Gastroenterology 134, 1424–1435 (2008).
Tang, W. et al. Multiple ‘omics’-analysis reveals the role of prostaglandin E2 in Hirschsprung’s disease. Free Radic. Biol. Med. 164, 390–398 (2021).
Wang, G. et al. Downregulation of microRNA-483-5p promotes cell proliferation and invasion by targeting GFRA4 in Hirschsprung’s disease. DNA Cell Biol. 36, 930–937 (2017).
Wu, F. et al. MPGES-1 derived PGE2 inhibits cell migration by regulating ARP2/3 in the pathogenesis of Hirschsprung disease. J. Pediatr. Surg. 54, 2032–2037 (2019).
Hong, M. et al. Runt-related transcription factor 1 promotes apoptosis and inhibits neuroblastoma progression in vitro and in vivo. J. Exp. Clin. Cancer Res. 39, 52 (2020).
Collins, T. J. ImageJ for microscopy. Biotechniques 43, 25–30 (2007).
Fattahi, F. et al. Deriving human ENS lineages for cell therapy and drug discovery in Hirschsprung disease. Nature 531, 105–109 (2016).
Guinard-Samuel, V. et al. Calretinin immunohistochemistry: a simple and efficient tool to diagnose Hirschsprung disease. Mod. Pathol. 22, 1379–1384 (2009).
Nabi, Z., Shava, U., Sekharan, A. & Nageshwar, R. D. Diagnosis of Hirschsprung’s disease in children: preliminary evaluation of a novel endoscopic technique for rectal biopsy. JGH Open 2, 322–326 (2018).
Moore, S. W. & Johnson, G. Acetylcholinesterase in Hirschsprung’s disease. Pediatr. Surg. Int. 21, 255–263 (2005).
Lv, X. et al. Molecular function predictions and diagnostic value analysis of plasma exosomal miRNAs in Hirschsprung’s disease. Epigenomics 12, 409–422 (2020).
Scaldaferri, F. et al. Gelatin tannate ameliorates acute colitis in mice by reinforcing mucus layer and modulating gut microbiota composition: emerging role for ‘gut barrier protectors’ in IBD? United Eur. Gastroenterol. J. 2, 113–122 (2014).
Leidinger, P., Backes, C., Meder, B., Meese, E. & Keller, A. The human miRNA repertoire of different blood compounds. BMC Genomics 15, 474 (2014).
Le, T. L. et al. Dysregulation of the NRG1/ERBB pathway causes a developmental disorder with gastrointestinal dysmotility in humans. J. Clin. Invest. 131, e146389 (2021).
Zhao, H. et al. miR-192/215-5p act as tumor suppressors and link Crohn’s disease and colorectal cancer by targeting common metabolic pathways: An integrated informatics analysis and experimental study. J. Cell. Physiol. 234, 21060–21075 (2019).
Li, P., Ou, Q., Braciak, T. A., Chen, G. & Oduncu, F. S. MicroRNA-192-5p is a predictive biomarker of survival for Stage IIIB colon cancer patients. Jpn J. Clin. Oncol. 48, 619–624 (2018).
Soret, R. et al. Glial cell-derived neurotrophic factor induces enteric neurogenesis and improves colon structure and function in mouse models of Hirschsprung disease. Gastroenterology 159, 1824–1838 (2020).
Watanabe, Y. et al. Differentiation of mouse enteric nervous system progenitor cells is controlled by endothelin 3 and requires regulation of Ednrb by SOX10 and ZEB2. Gastroenterology 152, 1139–1150 (2017).
Tong, Z., Cui, Q., Wang, J. & Zhou, Y. TransmiR v2.0: an updated transcription factor-microRNA regulation database. Nucleic Acids Res. 47, D253–D258 (2019).
Funding
This study was supported by the National Natural Science Foundation of China (81670511 and 81873848).
Author information
Authors and Affiliations
Contributions
M.H., X.L., and Y.L. carried out the RT-qPCR, CCK-8, western blot, luciferase reporter, and migration assays. X.L., Y.Z., Y.L., S.C., G.C., and S.L. helped with statistical analysis of the data. M.H. and S.T. designed the research and wrote the paper. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Hong, M., Li, X., Li, Y. et al. Hirschsprung’s disease: key microRNAs and target genes. Pediatr Res 92, 737–747 (2022). https://doi.org/10.1038/s41390-021-01872-1
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-021-01872-1
This article is cited by
-
Efficacy and potential toxicity of Duhuo Qinjiao Decoction in osteoarthritis via the NF-κB pathway
Clinical Rheumatology (2026)
-
circANKRD12/circTIMMDC1 synergistically regulates enteric neural crest cell migration via miR-181b-5p-PROX1-NOTCH1 axis in Hirschsprung’s disease
Pediatric Research (2025)
-
Exploring the diagnostic potential of plasma circ-CCDC66 in colorectal cancer
Scientific Reports (2025)
-
Downregulation of miR-144 blocked the proliferation and invasion of nerve cells in Hirschsprung disease by regulating Transcription Factor AP 4 (TFAP4)
Pediatric Surgery International (2023)
-
Plasma exosomal miR-199a-3p downregulates cell proliferation and migration in Hirschsprung’s disease by targeting mTOR
Pediatric Surgery International (2022)