Abstract
Background
Brain injury is a serious and common complication of critical congenital heart disease (CHD). Impaired autonomic development (assessed by heart rate variability (HRV)) is associated with brain injury in other high-risk neonatal populations.
Objective
To determine whether impaired early neonatal HRV is associated with pre-operative brain injury in CHD.
Methods
In infants with critical CHD, we evaluated HRV during the first 24 h of cardiac ICU (CICU) admission using time-domain (RMS 1, RMS 2, and alpha 1) and frequency-domain metrics (LF, nLF, HF, nHF). Pre-operative brain magnetic resonance imaging (MRI) was scored for injury using an established system. Spearman’s correlation coefficient was used to determine the association between HRV and pre-operative brain injury.
Results
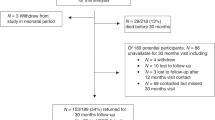
We enrolled 34 infants with median birth gestational age of 38.8 weeks (IQR 38.1–39.1). Median postnatal age at pre-operative brain MRI was 2 days (IQR 1–3 days). Thirteen infants had MRI evidence of brain injury. RMS 1 and RMS 2 were inversely correlated with pre-operative brain injury.
Conclusions
Time-domain metrics of autonomic function measured within the first 24 h of admission to the CICU are associated with pre-operative brain injury, and may perform better than frequency-domain metrics under non-stationary conditions such as critical illness.
Impact
-
Autonomic dysfunction, measured by heart rate variability (HRV), in early transition is associated with pre-operative brain injury in neonates with critical congenital heart disease.
-
These data extend our earlier findings by providing further evidence for (i) autonomic dysfunction in infants with CHD, and (ii) an association between autonomic dysfunction and brain injury in critically ill neonates.
-
These data support the notion that further investigation of HRV as a biomarker for brain injury risk is warranted in infants with critical CHD.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Grech, V. & Gatt, M. Syndromes and malformations associated with congenital heart disease in a population-based study. Int. J. Cardiol. 68, 151–156 (1999).
Tennstedt, C., Chaoui, R., Korner, H. & Dietel, M. Spectrum of congenital heart defects and extracardiac malformations associated with chromosomal abnormalities: results of a seven year necropsy study. Heart 82, 34–39 (1999).
Donofrio, M. T., Duplessis, A. J. & Limperopoulos, C. Impact of congenital heart disease on fetal brain development and injury. Curr. Opin. Pediatr. 23, 502–511 (2011).
Shillingford, A. J. et al. Aortic morphometry and microcephaly in hypoplastic left heart syndrome. Cardiol. Young. 17, 189–195 (2007).
Licht, D. J. et al. Brain maturation is delayed in infants with complex congenital heart defects. J. Thorac. Cardiovasc Surg. 137, 529–536 (2009). Discussion 536–527.
Miller, S. P. et al. Abnormal brain development in newborns with congenital heart disease. N. Engl. J. Med. 357, 1928–1938 (2007).
Sarajuuri, A. et al. Neurodevelopmental burden at age 5 years in patients with univentricular heart. Pediatrics 130, e1636–e1646 (2012).
Mulkey, S. B. et al. Multi-tiered analysis of brain injury in neonates with congenital heart disease. Pediatr. Cardiol. 34, 1772–1784 (2013).
Limperopoulos, C. et al. Neurodevelopmental status of newborns and infants with congenital heart defects before and after open heart surgery. J. Pediatr. 137, 638–645 (2000).
Limperopoulos, C. et al. Neurologic status of newborns with congenital heart defects before open heart surgery. Pediatrics 103, 402–408 (1999).
Donofrio, M. T. & Massaro, A. N. Impact of congenital heart disease on brain development and neurodevelopmental outcome. Int. J. Pediatr. 2010, 359390 (2010).
Mahle, W. T. et al. An MRI study of neurological injury before and after congenital heart surgery. Circulation 106, I109–I114 (2002).
Beca, J. et al. New white matter brain injury after infant heart surgery is associated with diagnostic group and the use of circulatory arrest. Circulation 127, 971–979 (2013).
Dimitropoulos, A. et al. Brain injury and development in newborns with critical congenital heart disease. Neurology 81, 241–248 (2013).
Ortinau, C. et al. Regional alterations in cerebral growth exist preoperatively in infants with congenital heart disease. J. Thorac. Cardiovasc. Surg. 143, 1264–1270 (2012).
Sethi, V. et al. Single-ventricle anatomy predicts delayed microstructural brain development. Pediatr. Res. 73, 661–667 (2013).
Petit, C. J. et al. Preoperative brain injury in transposition of the great arteries is associated with oxygenation and time to surgery, not balloon atrial septostomy. Circulation 119, 709–716 (2009).
Heart Rate Variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur. Heart J. 17, 354–381 (1996).
Al-Shargabi, T. et al. Changes in autonomic tone in premature infants developing necrotizing enterocolitis. Am. J. Perinatol. 35, 1079–1086 (2018).
Doheny, K. K. et al. Diminished vagal tone is a predictive biomarker of necrotizing enterocolitis-risk in preterm infants. Neurogastroenterol. Motil. 26, 832–840 (2014).
Vergales, B. D. et al. Depressed heart rate variability is associated with abnormal EEG, MRI, and death in neonates with hypoxic ischemic encephalopathy. Am. J. Perinatol. 31, 855–862 (2014).
Stone, M. L. et al. Abnormal heart rate characteristics before clinical diagnosis of necrotizing enterocolitis. J. Perinatol. 33, 847–850 (2013).
Fairchild, K. D. & Aschner, J. L. Hero monitoring to reduce mortality in NICU patients. Res. Rep. Neonatol. 2, 65–76 (2012).
Mulkey, S. B. et al. Heart rate variability is depressed in the early transitional period for newborns with complex congenital heart disease. Clin. Auton. Res. 30, 165–172 (2020).
Porges, S. W. & Furman, S. A. The early development of the autonomic nervous system provides a neural platform for social behavior: a polyvagal perspective. Infant Child Dev. 20, 106–118 (2011).
Schlatterer, S. D. & du Plessis, A. J. Exposures influencing the developing central autonomic nervous system. Birth Defects Res. 113, 845–863 (2021).
Malliani, A., Lombardi, F. & Pagani, M. Power spectrum analysis of heart rate variability: a tool to explore neural regulatory mechanisms. Br. Heart J. 71, 1–2 (1994).
Valenza, G., Citi, L., Saul, J. P. & Barbieri, R. Measures of sympathetic and parasympathetic autonomic outflow from heartbeat dynamics. J. Appl. Physiol. 125, 19–39 (2018).
Schlatterer, S. D. et al. Autonomic development in preterm infants is associated with morbidity of prematurity. Pediatr. Res. 2, 1–7 (2021). https://doi.org/10.1038/s41390-021-01420-x. Epub ahead of print.
Mulkey, S. B. et al. Autonomic nervous system maturation in the premature extrauterine milieu. Pediatr. Res. 89, 863–868 (2021).
Zun, Z. & Limperopoulos, C. Placental perfusion imaging using velocity-selective arterial spin labeling. Magn. Reson. Med. 80, 1036–1047 (2018).
De Asis-Cruz, J., Donofrio, M. T., Vezina, G. & Limperopoulos, C. Aberrant brain functional connectivity in newborns with congenital heart disease before cardiac surgery. Neuroimage. Clin. 17, 31–42 (2018).
Andropoulos, D. B. et al. Brain immaturity is associated with brain injury before and after neonatal cardiac surgery with high-flow bypass and cerebral oxygenation monitoring. J. Thorac. Cardiovasc. Surg. 139, 543–556 (2010).
Ulusar, U. D. et al. Adaptive rule based fetal QRS complex detection using Hilbert transform. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2009, 4666–4669 (2009).
Kota, S. et al. Identification of QRS complex in non-stationary electrocardiogram of sick infants. Computers Biol. Med. 87, 211–216 (2017).
Ulusar, U. D. et al. Adaptive rule based fetal QRS complex detection using Hilbert transform. Conf. Proc. IEEE Eng. Med Biol. Soc. 1, 4666–4669 (2009).
Govindan, R. B. Detrended fluctuation analysis using orthogonal polynomials. Phys. Rev. E 101, 010201 (2020).
Govindan, R. B., Preissl, H., Eswaran, H., Campbell, J. Q. & Lowery, C. L. Detrended fluctuation analysis of short data sets: ANA pplication to fetal cardiac. PhysD: Nonlinear Phenom. 226, 23–31 (2007).
Govindan, R. B. et al. Detrended fluctuation analysis of non-stationary cardiac beat-to-beat interval of sick infants. EPL 108, 40005–p40001–p40006 (2014).
Mulkey, S. B. et al. The effect of labor and delivery mode on electrocortical and brainstem autonomic function during neonatal transition. Sci. Rep. 9, 11020 (2019).
Montagna, A. & Nosarti, C. Socio-emotional development following very preterm birth: pathways to psychopathology. Front Psychol. 7, 80 (2016).
Hack, M., Schluchter, M., Cartar, L. & Rahman, M. Blood pressure among very low birth weight (<1.5 kg) young adults. Pediatr. Res. 58, 677–684 (2005).
Mulkey, S. B. & du Plessis, A. J. Autonomic nervous system development and its impact on neuropsychiatric outcome. Pediatr. Res. 85, 120–126 (2019).
Siddiqui, S., Fifer, W. P., Ordonez-Retamar, M., Nugent, J. D. & Williams, I. A. An antenatal marker of neurodevelopmental outcomes in infants with congenital heart disease. J. Perinatol. 37, 953–957 (2017).
Haraldsdottir, K. et al. Heart rate recovery after maximal exercise is impaired in healthy young adults born preterm. Eur. J. Appl. Physiol. 119, 857–866 (2019).
Thiriez, G. et al. Altered autonomic control in preterm newborns with impaired neurological outcomes. Clin. Auton. Res. 25, 233–242 (2015).
Fyfe, K. L., Yiallourou, S. R., Wong, F. Y. & Horne, R. S. The development of cardiovascular and cerebral vascular control in preterm infants. Sleep. Med. Rev. 18, 299–310 (2014).
Fyfe, K. L. et al. The effect of gestational age at birth on post-term maturation of heart rate variability. Sleep 38, 1635–1644 (2015).
Karin, J., Hirsch, M. & Akselrod, S. An estimate of fetal autonomic state by spectral analysis of fetal heart rate fluctuations. Pediatr. Res. 34, 134–138 (1993).
Porges, S. W. in The Polyvagal Theory (WW Norton & Company, 2011).
Longin, E., Gerstner, T., Schaible, T., Lenz, T. & Konig, S. Maturation of the autonomic nervous system: differences in heart rate variability in premature vs. term infants. J. Perinat. Med. 34, 303–308 (2006).
Clairambault, J., Curzi-Dascalova, L., Kauffmann, F., Medigue, C. & Leffler, C. Heart rate variability in normal sleeping full-term and preterm neonates. Early Hum. Dev. 28, 169–183 (1992).
Siddiqui, S. et al. Autonomic regulation in fetuses with congenital heart disease. Early Hum. Dev. 91, 195–198 (2015).
Limperopoulos, C. et al. Brain volume and metabolism in fetuses with congenital heart disease: evaluation with quantitative magnetic resonance imaging and spectroscopy. Circulation 121, 26–33 (2010).
Clouchoux, C. et al. Delayed cortical development in fetuses with complex congenital heart disease. Cereb. Cortex 23, 2932–2943 (2013).
McQuillen, P. S., Goff, D. A. & Licht, D. J. Effects of congenital heart disease on brain development. Prog. Pediatr. Cardiol. 29, 79–85 (2010).
Segar, J. L. Fetal and Neonatal Cardiovascular Physiology in Fetal and Neonatal Physiology (eds Polin, R. A., Fox, W. W. & Abman, S. H.) 789–793 (Elsevier, 2011).
Bevan, R. et al. Responsiveness of human infant cerebral arteries to sympathetic nerve stimulation and vasoactive agents. Pediatr. Res. 44, 730–739 (1998).
Hayashi, S., Park, M. K. & Kuehl, T. J. Higher sensitivity of cerebral arteries isolated from premature and newborn baboons to adrenergic and cholinergic stimulation. Life Sci. 35, 253–260 (1984).
Lou, H. C., Lassen, N. A. & Friis-Hansen, B. Impaired autoregulation of cerebral blood flow in the distressed newborn infant. J. Pediatr. 94, 118–121 (1979).
Kosiorek, A. et al. Predictors of neurological outcome following infant cardiac surgery without deep hypothermic circulatory arrest. Pediatr. Cardiol. (2021). Epub ahead of print.
Schlatterer, S. D. et al. Placental pathology and neuroimaging correlates in neonates with congenital heart disease. Sci. Rep. 9, 4137 (2019).
Andropoulos, D. B. et al. The association between brain injury, perioperative anesthetic exposure, and 12-month neurodevelopmental outcomes after neonatal cardiac surgery: a retrospective cohort study. Paediatr. Anaesth. 24, 266–274 (2014).
Rooks, V. J. et al. Prevalence and evolution of intracranial hemorrhage in asymptomatic term infants. AJNR Am. J. Neuroradiol. 29, 1082–1089 (2008).
Schneebaum Sender, N. et al. Effects of regional brain injury on the newborn autonomic nervous system. Early Hum. Dev. 90, 893–896 (2014).
Metzler, M. et al. Pattern of brain injury and depressed heart rate variability in newborns with hypoxic ischemic encephalopathy. Pediatr. Res. 82, 438–443 (2017).
Schneebaum Sender, N. et al. Cerebral modulation of the autonomic nervous system in term infants. J. Perinatol. 37, 558–562 (2017).
Funding
Grant support for this project is from the Canadian Institute of Health Research, MOP-8111 and National Institutes of Health: NHLBI R01 HL116585-01.
Author information
Authors and Affiliations
Contributions
S.D.S. designed the study, interpreted the data, and wrote the manuscript. R.B.G. performed HRV analysis for the study population and provided a critical review of the manuscript. J.M. interpreted and scored brain MRIs for brain injury and provided a critical review of the manuscript. S.D.B. performed statistical analyses for the study, interpreted the data, and provided a critical review of the manuscript. C.L. coordinated patient enrollment and clinical data acquisition and provided a critical review of the manuscript. M.T.D. interpreted patient echocardiograms, aided in study recruitment, and provided a critical review of the manuscript. S.B.M. provided a critical review of the manuscript and provided HRV data on subjects. C.L. designed the study with S.D.S. and A.J.d.P., provided MRI data on study subjects, and provided a critical review of the manuscript. A.J.d.P. designed the study and provided a critical review of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The Institutional Review Board of Children’s National Hospital approved this study and written informed consent was obtained from each participant.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Schlatterer, S.D., Govindan, R.B., Murnick, J. et al. In infants with congenital heart disease autonomic dysfunction is associated with pre-operative brain injury. Pediatr Res 91, 1723–1729 (2022). https://doi.org/10.1038/s41390-021-01931-7
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-021-01931-7