Abstract
Background
Neurodevelopmental abnormalities are prevalent in children with tetralogy of Fallot. Our aim was to investigate the structural brain alterations of preschool-aged children with tetralogy of Fallot and its correlation with neurodevelopmental outcome.
Methods
T1-weighted structural images were obtained from 25 children with tetralogy of Fallot who had undergone cardiopulmonary bypass surgery and from 24 normal controls. Cortical morphological indices including gray matter volume, cortical thickness, sulcal depth, gyrification, and cortical surface complexity were compared between the two groups. Neurodevelopmental assessments of the children with tetralogy of Fallot were performed with the Wechsler Preschool and Primary Scale of Intelligence.
Results
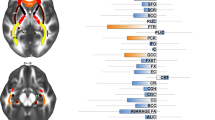
Cortical morphological differences between groups were distributed throughout the right caudal middle frontal gyrus, right fusiform gyrus, right lateral occipital gyrus, right precuneus, and left inferior parietal lobule. Among children with tetralogy of Fallot, altered cortical structures were correlated with the visual spatial index, working memory index, and perioperative variables.
Conclusion
Our results suggested that abnormal cortical structure in preschool-aged children with tetralogy of Fallot may be the persistent consequence of delayed cortical development in fetuses and cortical morphology can be used as an early potential biomarker to capture regional brain abnormalities that are relevant to neurodevelopmental outcomes.
Impact
-
Altered cortical structures in preschool-aged children with ToF were correlated with both neurodevelopmental outcomes and clinical risk factors.
-
Cortical morphology can be used as an effective tool to evaluate neuroanatomical changes and detect underlying neural mechanisms in ToF patients.
-
Abnormal cortical structure may be the continuous consequence of delayed fetal brain development in children with ToF.
Similar content being viewed by others

Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Bernier, P. L., Stefanescu, A., Samoukovic, G. & Tchervenkov, C. I. The challenge of congenital heart disease worldwide: epidemiologic and demographic facts. Semin. Thorac. Cardiovasc. Surg. Pediatr. Card. Surg. Annu. 13, 26–34 (2010).
Marelli, A., Miller, S. P., Marino, B. S., Jefferson, A. L. & Newburger, J. W. Brain in congenital heart disease across the lifespan: the cumulative burden of injury. Circulation 133, 1951–1962 (2016).
Karl, T. R. & Stocker, C. Tetralogy of Fallot and its variants. Pediatr. Crit. Care Med. 17, S330–S336 (2016).
Miller, S. P. et al. Abnormal brain development in newborns with congenital heart disease. N. Engl. J. Med. 357, 1928–1938 (2007).
Morton, S. U. et al. Abnormal left-hemispheric sulcal patterns correlate with neurodevelopmental outcomes in subjects with single ventricular congenital heart disease. Cereb. Cortex 30, 476–487 (2020).
Nagaraj, U. D. et al. Impaired global and regional cerebral perfusion in newborns with complex congenital heart disease. J. Pediatr. 167, 1018–1024 (2015).
Karmacharya, S. et al. Advanced diffusion imaging for assessing normal white matter development in neonates and characterizing aberrant development in congenital heart disease. Neuroimage. Clin. 19, 360–373 (2018).
King, T. Z. et al. fMRI investigation of working memory in adolescents with surgically treated congenital heart disease. Appl. Neuropsychol. Child. 6, 7–21 (2017).
Andropoulos, D. B. et al. Changing expectations for neurological outcomes after the neonatal arterial switch operation. Ann. Thorac. Surg. 94, 1250–1255 (2012).
Andropoulos, D. B. et al. The association between brain injury, perioperative anesthetic exposure, and 12-month neurodevelopmental outcomes after neonatal cardiac surgery: a retrospective cohort study. Paediatr. Anaesth. 24, 266–274 (2014).
Meuwly, E. et al. Postoperative brain volumes are associated with one-year neurodevelopmental outcome in children with severe congenital heart disease. Sci. Rep. 9, 10885 (2019).
Rollins, C. K. et al. White matter volume predicts language development in congenital heart disease. J. Pediatr. 181, 42.e2–48.e2 (2017).
Fontes, K. et al. Hippocampal alterations and functional correlates in adolescents and young adults with congenital heart disease. Hum. Brain Mapp. 40, 3548–3560 (2019).
Ehrler, M., Latal, B., Kretschmar, O., von Rhein, M. & O’Gorman Tuura, R. Altered frontal white matter microstructure is associated with working memory impairments in adolescents with congenital heart disease: a diffusion tensor imaging study. Neuroimage. Clin. 25, 102123 (2020).
Brewster, R. C., King, T. Z., Burns, T. G., Drossner, D. M. & Mahle, W. T. White matter integrity dissociates verbal memory and auditory attention span in emerging adults with congenital heart disease. J. Int. Neuropsychol. Soc. 21, 22–33 (2015).
Fumagalli, G. G. et al. Parieto-occipital sulcus widening differentiates posterior cortical atrophy from typical Alzheimer disease. Neuroimage. Clin. 28, 102453 (2020).
Si, F. F. et al. Cortical morphometric abnormality and its association with working memory in children with attention-deficit/hyperactivity disorder. Psychiatry Investig. 18, 679–687 (2021).
Otte, M. L. et al. Cortical morphology and illness insight in patients with schizophrenia. Eur. Arch. Psychiatry Clin. Neurosci. https://doi.org/10.1007/s00406-021-01328-x (2021).
Clouchoux, C. et al. Delayed cortical development in fetuses with complex congenital heart disease. Cereb. Cortex 23, 2932–2943 (2013).
Ortinau, C. M. et al. Early-emerging sulcal patterns are atypical in fetuses with congenital heart disease. Cereb. Cortex 29, 3605–3616 (2019).
Limperopoulos, C. et al. Brain volume and metabolism in fetuses with congenital heart disease: evaluation with quantitative magnetic resonance imaging and spectroscopy. Circulation 121, 26–33 (2010).
Kline, J. E. et al. Early cortical maturation predicts neurodevelopment in very preterm infants. Arch. Dis. Child. Fetal Neonatal Ed. 105, 460–465 (2020).
Claessens, N. H. et al. Delayed cortical gray matter development in neonates with severe congenital heart disease. Pediatr. Res. 80, 668–674 (2016).
Harley, E. M. et al. Engagement of fusiform cortex and disengagement of lateral occipital cortex in the acquisition of radiological expertise. Cereb. Cortex 19, 2746–2754 (2009).
Cassidy, A. R., Newburger, J. W. & Bellinger, D. C. Learning and memory in adolescents with critical biventricular congenital heart disease. J. Int. Neuropsychol. Soc. 23, 627–639 (2017).
Sandu, A. L. et al. Structural brain complexity and cognitive decline in late life–a longitudinal study in the Aberdeen 1936 birth cohort. Neuroimage 100, 558–563 (2014).
Gohel, S. et al. Resting-state functional connectivity of the middle frontal gyrus can predict language lateralization in patients with brain tumors. AJNR Am. J. Neuroradiol. 40, 319–325 (2019).
Ma, S. et al. Changes in cortical thickness are associated with cognitive ability in postoperative school-aged children with tetralogy of Fallot. Front. Neurol. 11, 691 (2020).
Norbom, L. B. et al. New insights into the dynamic development of the cerebral cortex in childhood and adolescence: integrating macro- and microstructural MRI findings. Prog. Neurobiol. 204, 102109 (2021).
Coupé, P., Catheline, G., Lanuza, E. & Manjón, J. V. Towards a unified analysis of brain maturation and aging across the entire lifespan: a MRI analysis. Hum. Brain Mapp. 38, 5501–5518 (2017).
Claessens, N. H. P., Khalili, N., Isgum, I. & Ter Heide, H. Brain and CSF volumes in fetuses and neonates with antenatal diagnosis of critical congenital heart disease: a longitudinal MRI study. AJNR Am. J. Neuroradiol. 40, 885–891 (2019).
Olshaker, H., Ber, R., Hoffman, D. & Derazne, E. Volumetric brain MRI study in fetuses with congenital heart disease. AJNR Am. J. Neuroradiol. 39, 1164–1169 (2018).
Peyvandi, S. et al. The association between cardiac physiology, acquired brain injury, and postnatal brain growth in critical congenital heart disease. J. Thorac. Cardiovasc. Surg. 155, 291.e3–300.e3 (2018).
Kuhn, V. A. et al. Determinants of neurological outcome in neonates with congenital heart disease following heart surgery. Pediatr. Res. 89, 1283–1290 (2021).
Funding
This project is funded by the Six Talent Peaks Project in Jiangsu Province (No. WSN-192), Jiangsu Commission of Health (No. LGY2019009), and Nanjing Municipal Science and Technology Bureau (No. 202002055).
Author information
Authors and Affiliations
Contributions
Study conceptualization and design: Ming Yang, X.M., Y. Liu. Data collection: Mingwen Yang, S.M., S.W., M.F., M.Z., Y. Li, S.C., Z.F. Data analysis and interpretation: Mingwen Yang, Y. Liu. Drafting the manuscript: Mingwen Yang. All authors contributed to the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Written informed consent for publication was obtained from all participants.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Yang, M., Liu, Y., Ma, S. et al. Altered brain structure in preschool-aged children with tetralogy of Fallot. Pediatr Res 93, 1321–1327 (2023). https://doi.org/10.1038/s41390-022-01987-z
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-022-01987-z
This article is cited by
-
Molecular insights into neurodevelopmental abnormalities and rescue mechanisms in the fetal prefrontal cortex with tetralogy of Fallot
Discover Neuroscience (2025)
-
Atoh1 mediated disturbance of neuronal maturation by perinatal hypoxia induces cognitive deficits
Communications Biology (2024)
-
Preoperative serum cortisone levels are associated with cognition in preschool-aged children with tetralogy of Fallot after corrective surgery: new evidence from human populations and mice
World Journal of Pediatrics (2024)