Abstract
Background
Neonates have high levels of cold-shock proteins (CSPs) in the normothermic brain for a limited period following birth. Hypoxic–ischemic (HI) insults in term infants produce neonatal encephalopathy (NE), and it remains unclear whether HI-induced pathology alters baseline CSP expression in the normothermic brain.
Methods
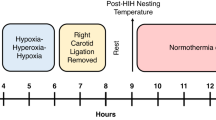
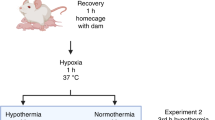
Here we established a version of the Rice–Vannucci model in PND 10 mice that incorporates rigorous temperature control.
Results
Common carotid artery (CCA)-ligation plus 25 min hypoxia (8% O2) in pups with targeted normothermia resulted in classic histopathological changes including increased hippocampal degeneration, astrogliosis, microgliosis, white matter changes, and cell signaling perturbations. Serial assessment of cortical, thalamic, and hippocampal RNA-binding motif 3 (RBM3), cold-inducible RNA binding protein (CIRBP), and reticulon-3 (RTN3) revealed a rapid age-dependent decrease in levels in sham and injured pups. CSPs were minimally affected by HI and the age point of lowest expression (PND 18) coincided with the timing at which heat-generating mechanisms mature in mice.
Conclusions
The findings suggest the need to determine whether optimized therapeutic hypothermia (depth and duration) can prevent the age-related decline in neuroprotective CSPs like RBM3 in the brain, and improve outcomes during critical phases of secondary injury and recovery after NE.
Impact
-
The rapid decrease in endogenous neuroprotective cold-shock proteins (CSPs) in the normothermic cortex, thalamus, and hippocampus from postnatal day (PND) 11–18, coincides with the timing of thermogenesis maturation in neonatal mice.
-
Hypoxia–ischemia (HI) has a minor impact on the normal age-dependent decline in brain CSP levels in neonates maintained normothermic post-injury.
-
HI robustly disrupts the expected correlation in RNA-binding motif 3 (RBM3) and reticulon-3 (RTN3).
-
The potent neuroprotectant RBM3 is not increased 1–4 days after HI in a mouse model of neonatal encephalopathy (NE) in the term newborn and in which rigorous temperature control prevents the manifestation of endogenous post-insult hypothermia.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Al-Fageeh, M. B. & Smales, C. M. Control and regulation of the cellular responses to cold shock: the responses in yeast and mammalian systems. Biochem. J. 397, 247–259 (2006).
Jackson, T. C. & Kochanek, P. M. A new vision for therapeutic hypothermia in the era of targeted temperature management: a speculative synthesis. Ther. Hypothermia Temp. Manag. 9, 13–47 (2019).
Chappell, S. A., Owens, G. C. & Mauro, V. P. A 5’ leader of Rbm3, a cold stress-induced mRNA, mediates internal initiation of translation with increased efficiency under conditions of mild hypothermia. J. Biol. Chem. 276, 36917–36922 (2001).
Al-Fageeh, M. B. & Smales, C. M. Cold-inducible RNA binding protein (CIRP) expression is modulated by alternative mRNAs. RNA 15, 1164–1176 (2009).
Bastide, A. et al. Rtn3 is a novel cold-induced protein and mediates neuroprotective effects of Rbm3. Curr. Biol. 27, 638–650 (2017).
Lujan, D. A., Ochoa, J. L. & Hartley, R. S. Cold-inducible RNA binding protein in cancer and inflammation. Wiley Interdiscip. Rev. RNA https://doi.org/10.1002/wrna.1462 (2018).
Qiang, X. et al. Cold-inducible RNA-binding protein (CIRP) triggers inflammatory responses in hemorrhagic shock and sepsis. Nat. Med. 19, 1489–1495 (2013).
Zhou, M., Yang, W. L., Ji, Y., Qiang, X. & Wang, P. Cold-inducible RNA-binding protein mediates neuroinflammation in cerebral ischemia. Biochim. Biophys. Acta 1840, 2253–2261 (2014).
Wong, J. J. et al. Rbm3 regulates temperature sensitive Mir-142-5p and Mir-143 (thermomirs), which target immune genes and control fever. Nucleic Acids Res 44, 2888–2897 (2016).
Liu, B. et al. The overexpression of Rbm3 alleviates TBI-induced behaviour impairment and AD-like tauopathy in mice. J. Cell. Mol. Med. 24, 9176–9188 (2020).
Peretti, D. et al. Rbm3 mediates structural plasticity and protective effects of cooling in neurodegeneration. Nature 518, 236–239 (2015).
Su, F. et al. CIRBP ameliorates neuronal amyloid toxicity via antioxidative and antiapoptotic pathways in primary cortical neurons. Oxid. Med. Cell. Longev. 2020, 2786139 (2020).
Sertel, S. M., von Elling-Tammen, M. S. & Rizzoli, S. O. The mRNA-binding protein Rbm3 regulates activity patterns and local synaptic translation in cultured hippocampal neurons. J. Neurosci. 41, 1157–1173 (2021).
Hu, X. et al. Transgenic mice overexpressing reticulon 3 develop neuritic abnormalities. EMBO J. 26, 2755–2767 (2007).
Kim, D. Y., Kim, K. M., Kim, E. J. & Jang, W. G. Hypothermia-induced RNA-binding motif protein 3 (Rbm3) stimulates osteoblast differentiation via the Erk signaling pathway. Biochem. Biophys. Res. Commun. 498, 459–465 (2018).
Cooper, S. T. et al. Effects of hibernation on bone marrow transcriptome in thirteen-lined ground squirrels. Physiol. Genomics 48, 513–525 (2016).
Dresios, J. et al. Cold stress-induced protein Rbm3 binds 60s ribosomal subunits, alters microrna levels, and enhances global protein synthesis. Proc. Natl Acad. Sci. USA 102, 1865–1870 (2005).
Elabbassi, E. B. et al. Head insulation and heat loss in naked and clothed newborns using a thermal mannequin. Med. Phys. 29, 1090–1096 (2002).
Baum, J. D. Keeping babies warm. Bull. Am. Coll. Nurse Midwives 16, 39–46 (1971).
Totapally, A. et al. Epidemiology and outcomes of children with accidental hypothermia: a propensity-matched study. J. Trauma Acute Care Surg. 82, 362–367 (2017).
Rosenthal, M., Poliquin, V. & Yu, A. Maternal hypothermia from environmental exposure in the third trimester. Int. J. Circumpolar Health 79, 1710894 (2020).
Pilotte, J., Cunningham, B. A., Edelman, G. M. & Vanderklish, P. W. Developmentally regulated expression of the cold-inducible RNA-binding motif protein 3 in euthermic rat brain. Brain Res. 1258, 12–24 (2009).
Xia, W., Su, L. & Jiao, J. Cold-induced protein Rbm3 orchestrates neurogenesis via modulating Yap mRNA stability in cold stress. J. Cell Biol. 217, 3464–3479 (2018).
Chip, S. et al. The RNA-binding protein Rbm3 is involved in hypothermia induced neuroprotection. Neurobiol. Dis. 43, 388–396 (2011).
Jackson, T. C., Kotermanski, S. E. & Kochanek, P. M. Infants uniquely express high levels of Rbm3 and other cold-adaptive neuroprotectant proteins in the human brain. Dev. Neurosci. 40, 325–336 (2018).
Jackson, T. C., Janesko-Feldman, K., Carlson, S. W., Kotermanski, S. E. & Kochanek, P. M. Robust Rbm3 and beta-Klotho expression in developing neurons in the human brain. J. Cereb. Blood Flow Metab. 39, 2355–2367 (2019).
Di Scala, F. et al. Tissue specificity and regulation of the N-terminal diversity of reticulon 3. Biochem. J. 385, 125–134 (2005).
Chakkarapani, A. A. et al. Therapies for neonatal encephalopathy: targeting the latent, secondary and tertiary phases of evolving brain injury. Semin. Fetal Neonatal Med. 26, 101256 (2021).
Marlow, N. et al. Neurological and developmental outcomes following neonatal encephalopathy treated with therapeutic hypothermia. Semin. Fetal Neonatal Med. 26, 101274 (2021).
Jacobs, S. E. et al. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst. Rev. CD003311 (2013).
Jacobs, S. E. et al. Whole-body hypothermia for term and near-term newborns with hypoxic-ischemic encephalopathy: a randomized controlled trial. Arch. Pediatr. Adolesc. Med. 165, 692–700 (2011).
El-Dib, M. et al. Should therapeutic hypothermia be offered to babies with mild neonatal encephalopathy in the first 6 h after birth? Pediatr. Res. 85, 442–448 (2019).
Hess, S. E. et al. Home improvement: C57bl/6j mice given more naturalistic nesting materials build better nests. J. Am. Assoc. Lab. Anim. Sci. 47, 25–31 (2008).
Gaskill, B. N. et al. Heat or insulation: behavioral titration of mouse preference for warmth or access to a nest. PLoS ONE 7, e32799 (2012).
Uchihara, T. Silver diagnosis in neuropathology: principles, practice and revised interpretation. Acta Neuropathol. 113, 483–499 (2007).
Semple, B. D., Blomgren, K., Gimlin, K., Ferriero, D. M. & Noble-Haeusslein, L. J. Brain development in rodents and humans: identifying benchmarks of maturation and vulnerability to injury across species. Prog. Neurobiol. 106-107, 1–16 (2013).
Reinboth, B. S. et al. Endogenous hypothermic response to hypoxia reduces brain injury: implications for modeling hypoxic-ischemic encephalopathy and therapeutic hypothermia in neonatal mice. Exp. Neurol. 283, 264–275 (2016).
Nakajima, W. et al. Apoptosis has a prolonged role in the neurodegeneration after hypoxic ischemia in the newborn rat. J. Neurosci. 20, 7994–8004 (2000).
Santagostino, S. F., Spinazzi, M. & Radaelli, E. Restricted sensitivity of Fj-C staining to assess neuronal degeneration and death in preclinical mouse studies. Vet. Pathol. 58, 643–649 (2021).
Meier, S. et al. The P75 neurotrophin receptor is required for the survival of neuronal progenitors and normal formation of the basal forebrain, striatum, thalamus and neocortex. Development 146, dev181933 (2019).
Sun, M. Y. et al. Bax inhibiting peptide reduces apoptosis in neonatal rat hypoxic-ischemic brain damage. Int. J. Clin. Exp. Pathol. 8, 14701–14708 (2015).
Annink, K. V. et al. The long-term effect of perinatal asphyxia on hippocampal volumes. Pediatr. Res. 85, 43–49 (2019).
Chalak, L. F. et al. Biomarkers for severity of neonatal hypoxic-ischemic encephalopathy and outcomes in newborns receiving hypothermia therapy. J. Pediatr. 164, 468.e1–474.e1 (2014).
Li, Y. et al. Osteopontin is a blood biomarker for microglial activation and brain injury in experimental hypoxic-ischemic encephalopathy. eNeuro 4, ENEURO.0253-16.2016 (2017).
Umekawa, T., Osman, A. M., Han, W., Ikeda, T. & Blomgren, K. Resident microglia, rather than blood-derived macrophages, contribute to the earlier and more pronounced inflammatory reaction in the immature compared with the adult hippocampus after hypoxia-ischemia. Glia 63, 2220–2230 (2015).
Shankaran, S. et al. Neonatal magnetic resonance imaging pattern of brain injury as a biomarker of childhood outcomes following a trial of hypothermia for neonatal hypoxic-ischemic encephalopathy. J. Pediatr. 167, 987.e3–993.e3 (2015).
Northington, F. J., Ferriero, D. M., Graham, E. M., Traystman, R. J. & Martin, L. J. Early neurodegeneration after hypoxia-ischemia in neonatal rat is necrosis while delayed neuronal death is apoptosis. Neurobiol. Dis. 8, 207–219 (2001).
Switzer, R. C. 3rd Application of silver degeneration stains for neurotoxicity testing. Toxicol. Pathol. 28, 70–83 (2000).
Gopagondanahalli, K. R. et al. Preterm hypoxic-ischemic encephalopathy. Front. Pediatr. 4, 114 (2016).
Volpe, J. J. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol. 8, 110–124 (2009).
Agut, T. et al. Preterm white matter injury: ultrasound diagnosis and classification. Pediatr. Res. 87, 37–49 (2020).
Lagerspetz, K. Y. H. Postnatal development of thermoregulation in laboratory mice. Helgoländer wissenschaftliche Meeresuntersuchungen 14, 559–571 (1966).
Wellmann, S. et al. Oxygen-regulated expression of the RNA-binding proteins RBM3 and CIRP by a HIF-1-independent mechanism. J. Cell Sci. 117, 1785–1794 (2004).
Zhu, X. et al. RBM3 promotes neurogenesis in a niche-dependent manner via IMP2-IGF2 signaling pathway after hypoxic-ischemic brain injury. Nat. Commun. 10, 3983 (2019).
Shankaran, S. et al. Effect of depth and duration of cooling on death or disability at age 18 months among neonates with hypoxic-ischemic encephalopathy: a randomized clinical trial. JAMA 318, 57–67 (2017).
Zhou, K. et al. Cold-inducible RNA-binding protein contributes to intracerebral hemorrhage-induced brain injury via TLR4 signaling. Brain Behav. 10, e01618 (2020).
Shi, Q., Hu, X., Prior, M. & Yan, R. The occurrence of aging-dependent reticulon 3 immunoreactive dystrophic neurites decreases cognitive function. J. Neurosci. 29, 5108–5115 (2009).
Hill, C. A. & Fitch, R. H. Sex differences in mechanisms and outcome of neonatal hypoxia-ischemia in rodent models: implications for sex-specific neuroprotection in clinical neonatal practice. Neurol. Res. Int. 2012, 867531 (2012).
Mirza, M. A., Ritzel, R., Xu, Y., McCullough, L. D. & Liu, F. Sexually dimorphic outcomes and inflammatory responses in hypoxic-ischemic encephalopathy. J. Neuroinflammation 12, 32 (2015).
Danno, S. et al. Increased transcript level of RBM3, a member of the glycine-rich rna-binding protein family, in human cells in response to cold stress. Biochem. Biophys. Res. Commun. 236, 804–807 (1997).
Zhang, Y. et al. Genes that escape X-inactivation in humans have high intraspecific variability in expression, are associated with mental impairment but are not slow evolving. Mol. Biol. Evol. 33, 302 (2016).
Wainer Katsir, K. & Linial, M. Human genes escaping X-inactivation revealed by single cell expression data. BMC Genomics 20, 201 (2019).
Dietz, R. M. et al. Therapeutic hypothermia protects against ischemia-induced impairment of synaptic plasticity following juvenile cardiac arrest in sex-dependent manner. Neuroscience 325, 132–141 (2016).
Back, S. A. White matter injury in the preterm infant: pathology and mechanisms. Acta Neuropathol. 134, 331–349 (2017).
Benninger, K. L. et al. Perspectives from the Society for Pediatric Research. Neonatal encephalopathy clinical trials: developing the future. Pediatr. Res. 89, 74–84 (2021).
Funding
This work was supported by NIH/NINDS grants R01NS105721 to T.C.J., by the University of South Florida Morsani College of Medicine start-up funds to T.C.J., by a Lloyd Reback Family Gift and T32 (2T32HD040686) to J.R.H., and by the Ake N. Grenvik Chair in Critical Care Medicine to P.M.K.
Author information
Authors and Affiliations
Contributions
T.C.J. conceived the study. T.C.J. and P.M.K. contributed to the study design. T.C.J. and J.R.H. drafted the manuscript. R.H.G., R.D.K., V.A.V., K.G., K.J.-F., and J.S. contributed to experiments and data acquisition. T.C.J., P.M.K., R.H.G., and J.R.H. contributed to data analysis. R.H.G., R.D.K., V.A.V., K.G., K.J.-F., and J.S. edited the draft and contributed to the final submitted version.
Corresponding author
Ethics declarations
Competing interests
T.C.J. and P.M.K. are co-inventors on a USPTO Application (No. 15/573,006) titled: “Method to Improve Neurologic Outcomes in Temperature Managed Patients.”
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Jackson, T.C., Herrmann, J.R., Garman, R.H. et al. Hypoxia–ischemia-mediated effects on neurodevelopmentally regulated cold-shock proteins in neonatal mice under strict temperature control. Pediatr Res (2022). https://doi.org/10.1038/s41390-022-01990-4
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41390-022-01990-4
This article is cited by
-
RBM3 Promotes Anti-inflammatory Responses in Microglia and Serves as a Neuroprotective Target of Ischemic Stroke
Molecular Neurobiology (2024)
-
Hypothermia increases cold-inducible protein expression and improves cerebellar-dependent learning after hypoxia ischemia in the neonatal rat
Pediatric Research (2023)
-
FGF21 modulates hippocampal cold-shock proteins and CA2-subregion proteins in neonatal mice with hypoxia–ischemia
Pediatric Research (2023)